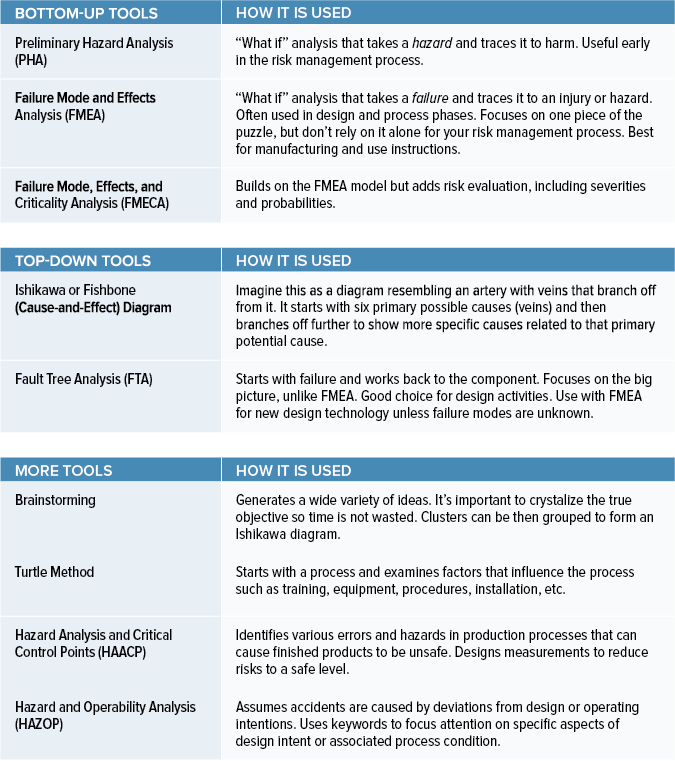
Medical Device Risk Analysis Example . Identify when to use risk. Discuss the reasons for conducting risk management activities for medical devices. Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european. Recognised by regulatory authorities such as those in the united states, europe, canada, and australia, iso 14971 holds a prominent status in. Risk analysis is an imperative step for all medical devices. What are the four different types? The one most people are familiar with is risk analysis associated with the design of a.
from www.orielstat.com
Risk analysis is an imperative step for all medical devices. What are the four different types? Discuss the reasons for conducting risk management activities for medical devices. Identify when to use risk. The one most people are familiar with is risk analysis associated with the design of a. Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european. Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. Recognised by regulatory authorities such as those in the united states, europe, canada, and australia, iso 14971 holds a prominent status in.
Medical Device Risk Management Analysis & Tools Oriel STAT A MATRIX
Medical Device Risk Analysis Example What are the four different types? Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. Recognised by regulatory authorities such as those in the united states, europe, canada, and australia, iso 14971 holds a prominent status in. Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. Discuss the reasons for conducting risk management activities for medical devices. Identify when to use risk. Risk analysis is an imperative step for all medical devices. What are the four different types? Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european. The one most people are familiar with is risk analysis associated with the design of a.
From qbd.eu
Why Medical Device Risk Management is as complex as it is crucial Medical Device Risk Analysis Example Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. Risk analysis is an imperative step for all medical devices. What are the four different types? Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. Risk management for medical devices performing risk. Medical Device Risk Analysis Example.
From www.mpo-mag.com
EMC Risk Management Files For Medical Device Developers Medical Medical Device Risk Analysis Example Recognised by regulatory authorities such as those in the united states, europe, canada, and australia, iso 14971 holds a prominent status in. What are the four different types? Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. Discuss the reasons for conducting risk management activities for medical. Medical Device Risk Analysis Example.
From sunstonepilot.com
The Big Picture for Medical Device Risk Management Sunstone Pilot, Inc. Medical Device Risk Analysis Example Risk analysis is an imperative step for all medical devices. Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. Identify when to use risk. What are the four different types? The one most people are familiar with is risk analysis associated with the design of a. Risk management for medical devices performing risk management. Medical Device Risk Analysis Example.
From qualcy.com
Medical Device Quality Risk Management · Qualcy eQMS Medical Device Risk Analysis Example Identify when to use risk. Discuss the reasons for conducting risk management activities for medical devices. Risk analysis is an imperative step for all medical devices. Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european. Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management. Medical Device Risk Analysis Example.
From spyro-soft.com
ISO 14971 Risk Management for Medical Devices explained Medical Device Risk Analysis Example The one most people are familiar with is risk analysis associated with the design of a. Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european. Discuss the reasons for conducting risk management activities for medical devices. What are the four different types? Identify when to use risk.. Medical Device Risk Analysis Example.
From medicaldevicehq.com
Crash course Project risk management for medical devices Medical Device Risk Analysis Example Identify when to use risk. What are the four different types? Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. Risk management for medical devices performing risk management became an essential requirement. Medical Device Risk Analysis Example.
From array.aami.org
Documenting Medical Device Risk Management through the Risk Medical Device Risk Analysis Example Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. The one most people are familiar with is risk analysis associated with the design of a. Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. Identify when to use risk. Risk management. Medical Device Risk Analysis Example.
From medicaldevicehq.com
FMEA vs ISO 14971 Medical Device HQ 1 Medical Device Risk Analysis Example Identify when to use risk. Risk analysis is an imperative step for all medical devices. Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european. Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. Discuss. Medical Device Risk Analysis Example.
From medicaldevicehq.com
Performing medical device risk evaluation Medical Device HQ 1 Medical Device Risk Analysis Example The one most people are familiar with is risk analysis associated with the design of a. Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european. What are the four different types? Risk. Medical Device Risk Analysis Example.
From blog.seerpharma.com
Application of ISO 14971 Risk Management to New Medical Devices Medical Device Risk Analysis Example The one most people are familiar with is risk analysis associated with the design of a. Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. Risk analysis is an imperative step for all medical devices. Discuss the reasons for conducting risk management activities for medical devices. Risk. Medical Device Risk Analysis Example.
From www.meddeviceonline.com
Managing Risk For Medical Device Clinical Trials Medical Device Risk Analysis Example Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european. What are the four different types? Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. Risk analysis is an imperative step for all medical devices. Discuss the reasons for conducting risk management. Medical Device Risk Analysis Example.
From shotnelo.weebly.com
Medical device risk assessment template shotnelo Medical Device Risk Analysis Example The one most people are familiar with is risk analysis associated with the design of a. Recognised by regulatory authorities such as those in the united states, europe, canada, and australia, iso 14971 holds a prominent status in. Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. Risk analysis is an imperative step for. Medical Device Risk Analysis Example.
From www.orielstat.com
Medical Device Risk Management Analysis & Tools Oriel STAT A MATRIX Medical Device Risk Analysis Example Discuss the reasons for conducting risk management activities for medical devices. Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. The one most people are familiar with is risk analysis associated with the design of a. Identify when to use risk. Risk analysis is the process of looking at a design or system and. Medical Device Risk Analysis Example.
From www.semanticscholar.org
Case study — Risk management for medical devices (based on ISO 14971 Medical Device Risk Analysis Example Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european. Discuss the reasons for conducting risk management activities for medical devices. The one most people are familiar with is risk analysis associated with the design of a. Identify when to use risk. What are the four different types?. Medical Device Risk Analysis Example.
From www.greenlight.guru
ISO 14971 Risk Management for Medical Devices [Guide] Medical Device Risk Analysis Example Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. Recognised by regulatory authorities such as those in the united states, europe, canada, and australia, iso 14971 holds a prominent status in. Risk analysis is an imperative step for all medical devices. Risk management for medical devices performing risk management became an essential requirement for. Medical Device Risk Analysis Example.
From hydrocodone6canadian6pharmacies6cod.blogspot.com
Iso14971 Risk Management Template / Creating A Medical Device Risk Medical Device Risk Analysis Example What are the four different types? Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. Discuss the reasons for conducting risk management activities for medical devices. The one most people are familiar with is risk analysis associated with the design of a. Learn about mdr/ivdr risk assessments. Medical Device Risk Analysis Example.
From kvalito.ch
Risk Management for Medical Devices ISO 149712019 Kvalito Medical Device Risk Analysis Example Identify when to use risk. Recognised by regulatory authorities such as those in the united states, europe, canada, and australia, iso 14971 holds a prominent status in. Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk. Medical Device Risk Analysis Example.
From www.slideserve.com
PPT Risk Management for Medical Devices PowerPoint Presentation, free Medical Device Risk Analysis Example Identify when to use risk. The one most people are familiar with is risk analysis associated with the design of a. Recognised by regulatory authorities such as those in the united states, europe, canada, and australia, iso 14971 holds a prominent status in. Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. Discuss the. Medical Device Risk Analysis Example.
From www.perforce.com
Condensed Guide to Medical Device Requirements Management Perforce Medical Device Risk Analysis Example Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. Identify when to use risk. Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european. Risk analysis is an imperative step for all medical devices. What. Medical Device Risk Analysis Example.
From medicaldevicehq.com
Risk management for medical device and ISO 149712019 infographic Medical Device Risk Analysis Example What are the four different types? Risk analysis is an imperative step for all medical devices. Recognised by regulatory authorities such as those in the united states, europe, canada, and australia, iso 14971 holds a prominent status in. Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european.. Medical Device Risk Analysis Example.
From www.researchgate.net
(PDF) Documenting Medical Device Risk Management through the Risk Medical Device Risk Analysis Example Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european. What are the four different types? Identify when to use risk. Discuss the reasons for conducting risk management activities for medical devices. The. Medical Device Risk Analysis Example.
From masoprealty.weebly.com
Medical device risk assessment template masoprealty Medical Device Risk Analysis Example The one most people are familiar with is risk analysis associated with the design of a. Discuss the reasons for conducting risk management activities for medical devices. Identify when to use risk. Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. Learn about mdr/ivdr risk assessments including. Medical Device Risk Analysis Example.
From mavink.com
Iso 14971 Risk Assessment Template Medical Device Risk Analysis Example Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. Identify when to use risk. Discuss the reasons for conducting risk management activities for medical devices. Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. Recognised by regulatory authorities such as those. Medical Device Risk Analysis Example.
From www.meddeviceonline.com
Managing Risk For Medical Device Clinical Trials Medical Device Risk Analysis Example Recognised by regulatory authorities such as those in the united states, europe, canada, and australia, iso 14971 holds a prominent status in. What are the four different types? Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european. The one most people are familiar with is risk analysis. Medical Device Risk Analysis Example.
From www.nsmedicaldevices.com
Risk Management / FMEA NS Medical Devices Medical Device Risk Analysis Example The one most people are familiar with is risk analysis associated with the design of a. Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european. Recognised by regulatory authorities such as those in the united states, europe, canada, and australia, iso 14971 holds a prominent status in.. Medical Device Risk Analysis Example.
From medicaldevicehq.com
Usability engineering and ISO 14971 risk management for medical devices Medical Device Risk Analysis Example Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. The one most people are familiar with is risk analysis associated with the design of a. Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european.. Medical Device Risk Analysis Example.
From www.meddeviceonline.com
Navigating The Universe Of Risk In Medical Device Development Medical Device Risk Analysis Example Identify when to use risk. Risk analysis is an imperative step for all medical devices. Discuss the reasons for conducting risk management activities for medical devices. What are the four different types? Recognised by regulatory authorities such as those in the united states, europe, canada, and australia, iso 14971 holds a prominent status in. Risk analysis is the process of. Medical Device Risk Analysis Example.
From www.orielstat.com
Creating a Medical Device Risk Management Plan and Doing Analysis Medical Device Risk Analysis Example Risk analysis is an imperative step for all medical devices. Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european. Recognised by regulatory authorities such as those in the united states, europe, canada, and australia, iso 14971 holds a prominent status in. Identify when to use risk. Risk. Medical Device Risk Analysis Example.
From www.youtube.com
Risk Management in the medical device industry in the EU YouTube Medical Device Risk Analysis Example What are the four different types? Discuss the reasons for conducting risk management activities for medical devices. Identify when to use risk. Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. The one most people are familiar with is risk analysis associated with the design of a.. Medical Device Risk Analysis Example.
From medicaldevicehq.com
How to integrate proactive safety by design with medical device risk Medical Device Risk Analysis Example Discuss the reasons for conducting risk management activities for medical devices. Recognised by regulatory authorities such as those in the united states, europe, canada, and australia, iso 14971 holds a prominent status in. Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. Identify when to use risk. The one most people are familiar with. Medical Device Risk Analysis Example.
From tsquality.ch
Risk Management Process ISO 14971 Risk Assessment Risk Control Medical Device Risk Analysis Example Recognised by regulatory authorities such as those in the united states, europe, canada, and australia, iso 14971 holds a prominent status in. The one most people are familiar with is risk analysis associated with the design of a. Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly.. Medical Device Risk Analysis Example.
From www.semanticscholar.org
Table 1 from Medical Device Safety Management Using Cybersecurity Risk Medical Device Risk Analysis Example What are the four different types? Identify when to use risk. Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. The one most people are familiar with is risk analysis associated with the design of a. Risk analysis is an imperative step for all medical devices. Discuss. Medical Device Risk Analysis Example.
From medicaldevicehq.com
Using MS Excel for medical device risk management Medical Device Risk Analysis Example Risk management for medical devices performing risk management became an essential requirement for medical device manufacturers with the publication of the european. The one most people are familiar with is risk analysis associated with the design of a. Risk analysis is an imperative step for all medical devices. What are the four different types? Learn about mdr/ivdr risk assessments including. Medical Device Risk Analysis Example.
From qualitydigest.com
Building a Practical Risk Management Process into Your QMS Quality Digest Medical Device Risk Analysis Example What are the four different types? Recognised by regulatory authorities such as those in the united states, europe, canada, and australia, iso 14971 holds a prominent status in. Discuss the reasons for conducting risk management activities for medical devices. The one most people are familiar with is risk analysis associated with the design of a. Risk analysis is an imperative. Medical Device Risk Analysis Example.
From www.greenlight.guru
Understanding ISO 14971 Medical Device Risk Management Medical Device Risk Analysis Example Learn about mdr/ivdr risk assessments including challenges, trends, and overall risk management for medical devices. Risk analysis is the process of looking at a design or system and actually coming up with possible hazards that could possibly. Discuss the reasons for conducting risk management activities for medical devices. Recognised by regulatory authorities such as those in the united states, europe,. Medical Device Risk Analysis Example.