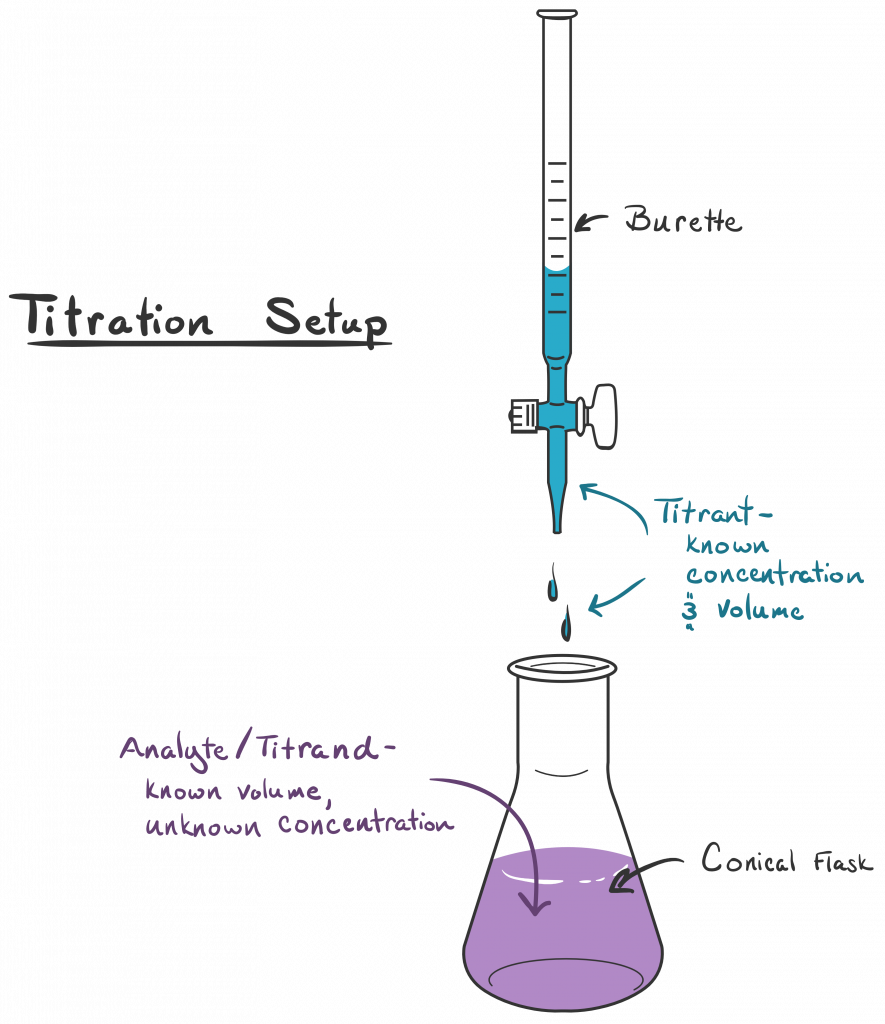
Tums Titration Lab . Antacids are weak bases, and. the parietal cells (the acid secreting cells) in the stomach secrete hydrochloric acid at a concentration of about 0.155 m; in this lab, you will determine the capacity of an antacid table to neutralize hydrochloric acid using a technique called “back titration.” in. Titration of an antacid objective: To measure the quantity of stomach acid that can be neutralized by a. in today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. the point of the titration is to measuring how much additional base (i.e. in this exercise, the method of titration will be used to determine the number of moles of \(\ce{h^+}\) neutralized per gram of antacid. Titration of a commercial antacid. Naoh) we have to add to neutralize the excess acid (i.e. In this experiment, you will standardize a solution of base using the analytical technique known as titration.
from www.microlit.com
Titration of a commercial antacid. To measure the quantity of stomach acid that can be neutralized by a. Naoh) we have to add to neutralize the excess acid (i.e. In this experiment, you will standardize a solution of base using the analytical technique known as titration. the parietal cells (the acid secreting cells) in the stomach secrete hydrochloric acid at a concentration of about 0.155 m; in this exercise, the method of titration will be used to determine the number of moles of \(\ce{h^+}\) neutralized per gram of antacid. Titration of an antacid objective: the point of the titration is to measuring how much additional base (i.e. Antacids are weak bases, and. in today’s lab you will analyze antacids which are common medications used to neutralize stomach acid.
An Advanced Guide to Titration Microlit
Tums Titration Lab Titration of an antacid objective: the point of the titration is to measuring how much additional base (i.e. In this experiment, you will standardize a solution of base using the analytical technique known as titration. in today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. Antacids are weak bases, and. Titration of a commercial antacid. in this exercise, the method of titration will be used to determine the number of moles of \(\ce{h^+}\) neutralized per gram of antacid. Titration of an antacid objective: in this lab, you will determine the capacity of an antacid table to neutralize hydrochloric acid using a technique called “back titration.” in. To measure the quantity of stomach acid that can be neutralized by a. Naoh) we have to add to neutralize the excess acid (i.e. the parietal cells (the acid secreting cells) in the stomach secrete hydrochloric acid at a concentration of about 0.155 m;
From parholikhotoacknoledge.blogspot.com
Titrations in Chemistry Unveiling the Secrets of Quantitative Analysis Tums Titration Lab in this exercise, the method of titration will be used to determine the number of moles of \(\ce{h^+}\) neutralized per gram of antacid. the parietal cells (the acid secreting cells) in the stomach secrete hydrochloric acid at a concentration of about 0.155 m; In this experiment, you will standardize a solution of base using the analytical technique known. Tums Titration Lab.
From lab5-6redoxtitration.weebly.com
Procedure Station 56 Redox Titration Lab Tums Titration Lab Titration of a commercial antacid. Naoh) we have to add to neutralize the excess acid (i.e. To measure the quantity of stomach acid that can be neutralized by a. Titration of an antacid objective: the point of the titration is to measuring how much additional base (i.e. in this exercise, the method of titration will be used to. Tums Titration Lab.
From mungfali.com
Titration Lab Diagram Tums Titration Lab Titration of an antacid objective: Antacids are weak bases, and. in today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. To measure the quantity of stomach acid that can be neutralized by a. the point of the titration is to measuring how much additional base (i.e. In this experiment, you will standardize. Tums Titration Lab.
From mycentennial.sd43.bc.ca
Titration Edublog Brandon’s Blog Tums Titration Lab To measure the quantity of stomach acid that can be neutralized by a. Titration of a commercial antacid. the parietal cells (the acid secreting cells) in the stomach secrete hydrochloric acid at a concentration of about 0.155 m; in today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. In this experiment, you. Tums Titration Lab.
From www.reagent.co.uk
Who Invented Titration? The Science Blog Tums Titration Lab Titration of an antacid objective: the point of the titration is to measuring how much additional base (i.e. Antacids are weak bases, and. Naoh) we have to add to neutralize the excess acid (i.e. the parietal cells (the acid secreting cells) in the stomach secrete hydrochloric acid at a concentration of about 0.155 m; To measure the quantity. Tums Titration Lab.
From www.microlit.com
An Advanced Guide to Titration Microlit Tums Titration Lab in today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. in this lab, you will determine the capacity of an antacid table to neutralize hydrochloric acid using a technique called “back titration.” in. Titration of a commercial antacid. Antacids are weak bases, and. Titration of an antacid objective: Naoh) we have to. Tums Titration Lab.
From www.youtube.com
Antacid Titration Experimental Set Up YouTube Tums Titration Lab in this exercise, the method of titration will be used to determine the number of moles of \(\ce{h^+}\) neutralized per gram of antacid. Antacids are weak bases, and. in today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. To measure the quantity of stomach acid that can be neutralized by a. Titration. Tums Titration Lab.
From www.flinnsci.com
AcidBase Titrations—Classic Lab Kit for AP® Chemistry Flinn Scientific Tums Titration Lab in this lab, you will determine the capacity of an antacid table to neutralize hydrochloric acid using a technique called “back titration.” in. the point of the titration is to measuring how much additional base (i.e. To measure the quantity of stomach acid that can be neutralized by a. Titration of an antacid objective: in this exercise,. Tums Titration Lab.
From www.youtube.com
AP Chemistry TUMS Lab Calculations Explained YouTube Tums Titration Lab In this experiment, you will standardize a solution of base using the analytical technique known as titration. the point of the titration is to measuring how much additional base (i.e. in this lab, you will determine the capacity of an antacid table to neutralize hydrochloric acid using a technique called “back titration.” in. To measure the quantity of. Tums Titration Lab.
From www.youtube.com
AP Chemistry Titration of Tums Lab setup YouTube Tums Titration Lab in this lab, you will determine the capacity of an antacid table to neutralize hydrochloric acid using a technique called “back titration.” in. In this experiment, you will standardize a solution of base using the analytical technique known as titration. the point of the titration is to measuring how much additional base (i.e. in this exercise, the. Tums Titration Lab.
From www.tes.com
Acid Base Titration Theory, Titration Lab, Titration Stoichiometry Tums Titration Lab Titration of an antacid objective: Naoh) we have to add to neutralize the excess acid (i.e. Antacids are weak bases, and. the point of the titration is to measuring how much additional base (i.e. Titration of a commercial antacid. in this exercise, the method of titration will be used to determine the number of moles of \(\ce{h^+}\) neutralized. Tums Titration Lab.
From chem.libretexts.org
AcidBase Titrations Chemistry LibreTexts Tums Titration Lab To measure the quantity of stomach acid that can be neutralized by a. In this experiment, you will standardize a solution of base using the analytical technique known as titration. Naoh) we have to add to neutralize the excess acid (i.e. the point of the titration is to measuring how much additional base (i.e. Titration of a commercial antacid.. Tums Titration Lab.
From beyondlabz.freshdesk.com
Titrations Lab Overview Beyond Labz Tums Titration Lab In this experiment, you will standardize a solution of base using the analytical technique known as titration. in today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. Naoh) we have to add to neutralize the excess acid (i.e. To measure the quantity of stomach acid that can be neutralized by a. the. Tums Titration Lab.
From betterlesson.com
Eleventh grade Lesson Titration Lab BetterLesson Tums Titration Lab the point of the titration is to measuring how much additional base (i.e. To measure the quantity of stomach acid that can be neutralized by a. the parietal cells (the acid secreting cells) in the stomach secrete hydrochloric acid at a concentration of about 0.155 m; in today’s lab you will analyze antacids which are common medications. Tums Titration Lab.
From www.scribd.com
Tums Lab Report Download Free PDF Titration Chemistry Tums Titration Lab To measure the quantity of stomach acid that can be neutralized by a. in this lab, you will determine the capacity of an antacid table to neutralize hydrochloric acid using a technique called “back titration.” in. Titration of a commercial antacid. In this experiment, you will standardize a solution of base using the analytical technique known as titration. Naoh). Tums Titration Lab.
From rebeccasouthtitrationlab.weebly.com
Pictures and Video Titration Lab Tums Titration Lab Titration of a commercial antacid. Antacids are weak bases, and. the point of the titration is to measuring how much additional base (i.e. in this exercise, the method of titration will be used to determine the number of moles of \(\ce{h^+}\) neutralized per gram of antacid. In this experiment, you will standardize a solution of base using the. Tums Titration Lab.
From studylib.net
Lab 7 Titration of Tums® Tums Titration Lab To measure the quantity of stomach acid that can be neutralized by a. in today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. Antacids are weak bases, and. in this exercise, the method of titration will be used to determine the number of moles of \(\ce{h^+}\) neutralized per gram of antacid. Titration. Tums Titration Lab.
From studylib.net
Experiment 11 Titration of a Commercial Antacid Tums Titration Lab Titration of an antacid objective: In this experiment, you will standardize a solution of base using the analytical technique known as titration. To measure the quantity of stomach acid that can be neutralized by a. Antacids are weak bases, and. Naoh) we have to add to neutralize the excess acid (i.e. in this exercise, the method of titration will. Tums Titration Lab.
From www.coursehero.com
A Titration Lab Station III IV In order to ensure that a colour Tums Titration Lab Naoh) we have to add to neutralize the excess acid (i.e. In this experiment, you will standardize a solution of base using the analytical technique known as titration. Titration of a commercial antacid. the parietal cells (the acid secreting cells) in the stomach secrete hydrochloric acid at a concentration of about 0.155 m; in this lab, you will. Tums Titration Lab.
From www.youtube.com
Antacid Lab Trial 1 Tums YouTube Tums Titration Lab the point of the titration is to measuring how much additional base (i.e. in today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. Titration of a commercial antacid. Titration of an antacid objective: To measure the quantity of stomach acid that can be neutralized by a. the parietal cells (the acid. Tums Titration Lab.
From www.youtube.com
Titrations lab YouTube Tums Titration Lab Titration of a commercial antacid. in this lab, you will determine the capacity of an antacid table to neutralize hydrochloric acid using a technique called “back titration.” in. In this experiment, you will standardize a solution of base using the analytical technique known as titration. Titration of an antacid objective: Naoh) we have to add to neutralize the excess. Tums Titration Lab.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Tums Titration Lab In this experiment, you will standardize a solution of base using the analytical technique known as titration. Titration of a commercial antacid. in this exercise, the method of titration will be used to determine the number of moles of \(\ce{h^+}\) neutralized per gram of antacid. Titration of an antacid objective: the parietal cells (the acid secreting cells) in. Tums Titration Lab.
From ctdvdimri.blogspot.com
Chemistry Honors Blog Lab 14 Titration Lab Tums Titration Lab To measure the quantity of stomach acid that can be neutralized by a. Titration of a commercial antacid. Naoh) we have to add to neutralize the excess acid (i.e. in this exercise, the method of titration will be used to determine the number of moles of \(\ce{h^+}\) neutralized per gram of antacid. Titration of an antacid objective: the. Tums Titration Lab.
From studylib.net
Titration Lab Tums Titration Lab in this exercise, the method of titration will be used to determine the number of moles of \(\ce{h^+}\) neutralized per gram of antacid. To measure the quantity of stomach acid that can be neutralized by a. Antacids are weak bases, and. Titration of a commercial antacid. the point of the titration is to measuring how much additional base. Tums Titration Lab.
From www.studocu.com
Titration Lab Sheet What does this tell you about the substance? it’s Tums Titration Lab the point of the titration is to measuring how much additional base (i.e. the parietal cells (the acid secreting cells) in the stomach secrete hydrochloric acid at a concentration of about 0.155 m; in today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. To measure the quantity of stomach acid that. Tums Titration Lab.
From www.youtube.com
Experiment 7 OxidationReduction Titrations SMU Chemistry YouTube Tums Titration Lab Titration of an antacid objective: in this exercise, the method of titration will be used to determine the number of moles of \(\ce{h^+}\) neutralized per gram of antacid. in today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. Titration of a commercial antacid. the parietal cells (the acid secreting cells) in. Tums Titration Lab.
From www.youtube.com
Solution Stoichiometry Titrations Lab YouTube Tums Titration Lab Titration of an antacid objective: in this lab, you will determine the capacity of an antacid table to neutralize hydrochloric acid using a technique called “back titration.” in. To measure the quantity of stomach acid that can be neutralized by a. the parietal cells (the acid secreting cells) in the stomach secrete hydrochloric acid at a concentration of. Tums Titration Lab.
From ck12.org
Titration CK12 Foundation Tums Titration Lab Antacids are weak bases, and. the parietal cells (the acid secreting cells) in the stomach secrete hydrochloric acid at a concentration of about 0.155 m; in this lab, you will determine the capacity of an antacid table to neutralize hydrochloric acid using a technique called “back titration.” in. the point of the titration is to measuring how. Tums Titration Lab.
From chemistry.analia-sanchez.net
Titration Lab Chemistry Classes / Ronald Reagan S.H.S. Tums Titration Lab Antacids are weak bases, and. the point of the titration is to measuring how much additional base (i.e. in today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. Titration of an antacid objective: Titration of a commercial antacid. in this lab, you will determine the capacity of an antacid table to. Tums Titration Lab.
From mmerevise.co.uk
Titrations and Uncertainties MME Tums Titration Lab Titration of a commercial antacid. Antacids are weak bases, and. Titration of an antacid objective: Naoh) we have to add to neutralize the excess acid (i.e. the point of the titration is to measuring how much additional base (i.e. In this experiment, you will standardize a solution of base using the analytical technique known as titration. in this. Tums Titration Lab.
From www.chemedx.org
How do antacids work? Titration of an Antacid Chemical Education Xchange Tums Titration Lab Antacids are weak bases, and. Titration of an antacid objective: Naoh) we have to add to neutralize the excess acid (i.e. the point of the titration is to measuring how much additional base (i.e. in today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. Titration of a commercial antacid. To measure the. Tums Titration Lab.
From ctdvdimri.blogspot.com
Chemistry Honors Blog Lab 14 Titration Lab Tums Titration Lab in this lab, you will determine the capacity of an antacid table to neutralize hydrochloric acid using a technique called “back titration.” in. Titration of a commercial antacid. the point of the titration is to measuring how much additional base (i.e. in this exercise, the method of titration will be used to determine the number of moles. Tums Titration Lab.
From mycentennial.sd43.bc.ca
Titration lab Chem 11 Alexandra’s Blog Tums Titration Lab In this experiment, you will standardize a solution of base using the analytical technique known as titration. in this exercise, the method of titration will be used to determine the number of moles of \(\ce{h^+}\) neutralized per gram of antacid. Naoh) we have to add to neutralize the excess acid (i.e. Titration of a commercial antacid. Titration of an. Tums Titration Lab.
From www.alamy.com
Scientist performing titration in a chemistry laboratory with Redox Tums Titration Lab Titration of an antacid objective: To measure the quantity of stomach acid that can be neutralized by a. Titration of a commercial antacid. In this experiment, you will standardize a solution of base using the analytical technique known as titration. Naoh) we have to add to neutralize the excess acid (i.e. in this lab, you will determine the capacity. Tums Titration Lab.
From www.youtube.com
TUMS LAB Calculations Review YouTube Tums Titration Lab the parietal cells (the acid secreting cells) in the stomach secrete hydrochloric acid at a concentration of about 0.155 m; In this experiment, you will standardize a solution of base using the analytical technique known as titration. in this exercise, the method of titration will be used to determine the number of moles of \(\ce{h^+}\) neutralized per gram. Tums Titration Lab.