Copper Ii Sulfate Electrolysis Products . Electrolysis involves using electricity to break. Use this demonstration to find the value of the faraday constant from electrolysis of copper (ii) sulfate solution. In this experiment, students learn how the value of the faraday. Electrolysis of copper sulfate (cuso 4) solution. Copper sulfate is soluble in water and gives a blue colour aqueous solution though anhydrous copper sulfate solid is white. Using the simple apparatus (above left diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis of copper sulfate solution are (i) a. Pour copper (ii) sulfate solution into. Explore the electrolysis of copper(ii) sulfate solution and related industrial processes with this class experiment. Electrolysis of copper (ii) sulfate using graphite electrodes. Includes kit list and safety instructions. This experiment is designed to demonstrate the different products obtained when the electrolysis of copper(ii) sulfate solution is carried out first. There are different observations for each electrode.
from www.doubtnut.com
In this experiment, students learn how the value of the faraday. Electrolysis of copper sulfate (cuso 4) solution. Electrolysis involves using electricity to break. Pour copper (ii) sulfate solution into. Electrolysis of copper (ii) sulfate using graphite electrodes. Includes kit list and safety instructions. Explore the electrolysis of copper(ii) sulfate solution and related industrial processes with this class experiment. Use this demonstration to find the value of the faraday constant from electrolysis of copper (ii) sulfate solution. Copper sulfate is soluble in water and gives a blue colour aqueous solution though anhydrous copper sulfate solid is white. There are different observations for each electrode.
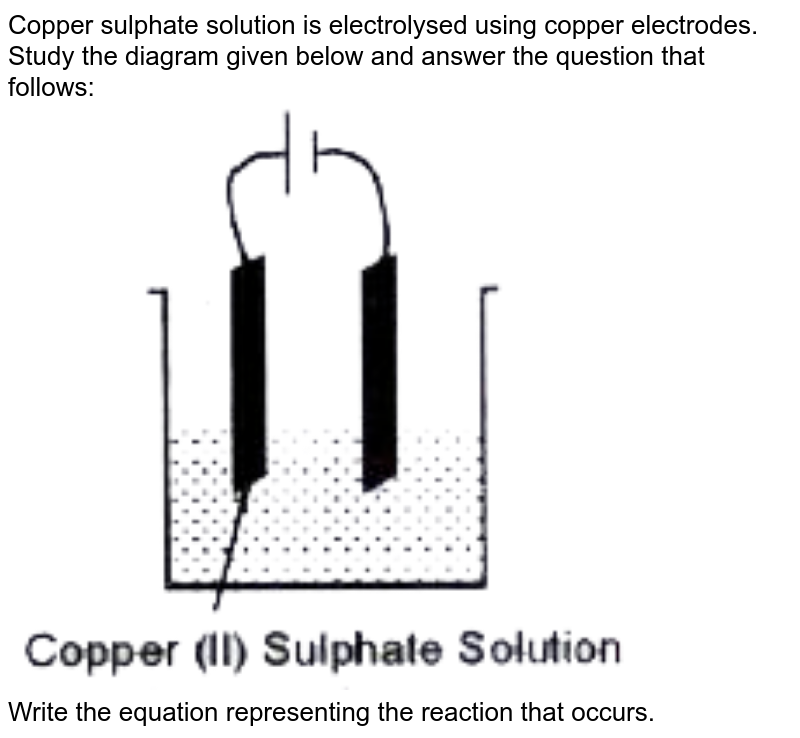
Copper sulphate solution is electrolysed using copper electrodes.
Copper Ii Sulfate Electrolysis Products Electrolysis involves using electricity to break. Electrolysis involves using electricity to break. Copper sulfate is soluble in water and gives a blue colour aqueous solution though anhydrous copper sulfate solid is white. Electrolysis of copper (ii) sulfate using graphite electrodes. Using the simple apparatus (above left diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis of copper sulfate solution are (i) a. This experiment is designed to demonstrate the different products obtained when the electrolysis of copper(ii) sulfate solution is carried out first. In this experiment, students learn how the value of the faraday. Electrolysis of copper sulfate (cuso 4) solution. Pour copper (ii) sulfate solution into. Includes kit list and safety instructions. There are different observations for each electrode. Explore the electrolysis of copper(ii) sulfate solution and related industrial processes with this class experiment. Use this demonstration to find the value of the faraday constant from electrolysis of copper (ii) sulfate solution.
From ar.inspiredpencil.com
Copper Sulfate Solution Electrolysis Copper Ii Sulfate Electrolysis Products Pour copper (ii) sulfate solution into. Electrolysis of copper (ii) sulfate using graphite electrodes. Copper sulfate is soluble in water and gives a blue colour aqueous solution though anhydrous copper sulfate solid is white. Electrolysis involves using electricity to break. Using the simple apparatus (above left diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of. Copper Ii Sulfate Electrolysis Products.
From icsechemistry16.blogspot.com
Electrolysis of copper sulphate using copper electrodes Copper Ii Sulfate Electrolysis Products Electrolysis of copper (ii) sulfate using graphite electrodes. There are different observations for each electrode. Electrolysis involves using electricity to break. This experiment is designed to demonstrate the different products obtained when the electrolysis of copper(ii) sulfate solution is carried out first. Explore the electrolysis of copper(ii) sulfate solution and related industrial processes with this class experiment. Copper sulfate is. Copper Ii Sulfate Electrolysis Products.
From fineartamerica.com
Electrolysis Of Copper Sulphate Photograph by Trevor Clifford Photography Copper Ii Sulfate Electrolysis Products This experiment is designed to demonstrate the different products obtained when the electrolysis of copper(ii) sulfate solution is carried out first. In this experiment, students learn how the value of the faraday. Includes kit list and safety instructions. Electrolysis involves using electricity to break. Copper sulfate is soluble in water and gives a blue colour aqueous solution though anhydrous copper. Copper Ii Sulfate Electrolysis Products.
From www.sciencephoto.com
Copper sulphate electrolysis Stock Image C025/6915 Science Photo Copper Ii Sulfate Electrolysis Products Copper sulfate is soluble in water and gives a blue colour aqueous solution though anhydrous copper sulfate solid is white. Using the simple apparatus (above left diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis of copper sulfate solution are (i) a. Electrolysis of copper sulfate (cuso 4) solution. There are different observations. Copper Ii Sulfate Electrolysis Products.
From nationaldefensepac.org
Principle Of Electrolysis Of Copper Sulfate Electrolyte, 55 OFF Copper Ii Sulfate Electrolysis Products Electrolysis of copper (ii) sulfate using graphite electrodes. This experiment is designed to demonstrate the different products obtained when the electrolysis of copper(ii) sulfate solution is carried out first. Electrolysis of copper sulfate (cuso 4) solution. Use this demonstration to find the value of the faraday constant from electrolysis of copper (ii) sulfate solution. Explore the electrolysis of copper(ii) sulfate. Copper Ii Sulfate Electrolysis Products.
From www.youtube.com
Electrolysis of Aqueous Copper ii Sulfate using Carbon Electrodes YouTube Copper Ii Sulfate Electrolysis Products Use this demonstration to find the value of the faraday constant from electrolysis of copper (ii) sulfate solution. Electrolysis involves using electricity to break. Copper sulfate is soluble in water and gives a blue colour aqueous solution though anhydrous copper sulfate solid is white. Electrolysis of copper (ii) sulfate using graphite electrodes. Explore the electrolysis of copper(ii) sulfate solution and. Copper Ii Sulfate Electrolysis Products.
From gioulvood.blob.core.windows.net
Electrolysis Of Copper Ii Chloride Using Carbon Electrodes at Willie Copper Ii Sulfate Electrolysis Products Using the simple apparatus (above left diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis of copper sulfate solution are (i) a. There are different observations for each electrode. This experiment is designed to demonstrate the different products obtained when the electrolysis of copper(ii) sulfate solution is carried out first. Electrolysis of copper. Copper Ii Sulfate Electrolysis Products.
From gioulvood.blob.core.windows.net
Electrolysis Of Copper Ii Chloride Using Carbon Electrodes at Willie Copper Ii Sulfate Electrolysis Products In this experiment, students learn how the value of the faraday. Electrolysis of copper sulfate (cuso 4) solution. Pour copper (ii) sulfate solution into. There are different observations for each electrode. Electrolysis involves using electricity to break. Copper sulfate is soluble in water and gives a blue colour aqueous solution though anhydrous copper sulfate solid is white. This experiment is. Copper Ii Sulfate Electrolysis Products.
From www.dreamstime.com
Electroplating with Copper Using Copper Sulfate Electrolyte Stock Copper Ii Sulfate Electrolysis Products Includes kit list and safety instructions. Copper sulfate is soluble in water and gives a blue colour aqueous solution though anhydrous copper sulfate solid is white. Using the simple apparatus (above left diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis of copper sulfate solution are (i) a. There are different observations for. Copper Ii Sulfate Electrolysis Products.
From askfilo.com
III. Electrolysis of copper sulphate solution using platinum anode and co.. Copper Ii Sulfate Electrolysis Products Explore the electrolysis of copper(ii) sulfate solution and related industrial processes with this class experiment. There are different observations for each electrode. Pour copper (ii) sulfate solution into. Electrolysis of copper sulfate (cuso 4) solution. Use this demonstration to find the value of the faraday constant from electrolysis of copper (ii) sulfate solution. Electrolysis of copper (ii) sulfate using graphite. Copper Ii Sulfate Electrolysis Products.
From chemistrylabs-2.blogspot.com
Electrolysis Of Copper Sulfate Solution Chemistry Labs Copper Ii Sulfate Electrolysis Products Use this demonstration to find the value of the faraday constant from electrolysis of copper (ii) sulfate solution. Pour copper (ii) sulfate solution into. There are different observations for each electrode. Electrolysis of copper sulfate (cuso 4) solution. Using the simple apparatus (above left diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis. Copper Ii Sulfate Electrolysis Products.
From courses.lumenlearning.com
Predicting the Products of Electrolysis Introduction to Chemistry Copper Ii Sulfate Electrolysis Products Using the simple apparatus (above left diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis of copper sulfate solution are (i) a. Electrolysis of copper sulfate (cuso 4) solution. Explore the electrolysis of copper(ii) sulfate solution and related industrial processes with this class experiment. Includes kit list and safety instructions. In this experiment,. Copper Ii Sulfate Electrolysis Products.
From ar.inspiredpencil.com
Copper Electrolytic Cell Copper Ii Sulfate Electrolysis Products Use this demonstration to find the value of the faraday constant from electrolysis of copper (ii) sulfate solution. There are different observations for each electrode. Includes kit list and safety instructions. Pour copper (ii) sulfate solution into. Electrolysis of copper (ii) sulfate using graphite electrodes. Copper sulfate is soluble in water and gives a blue colour aqueous solution though anhydrous. Copper Ii Sulfate Electrolysis Products.
From www.youtube.com
AQA Required Practical The electrolysis of copper (II) chloride Copper Ii Sulfate Electrolysis Products In this experiment, students learn how the value of the faraday. Includes kit list and safety instructions. There are different observations for each electrode. Copper sulfate is soluble in water and gives a blue colour aqueous solution though anhydrous copper sulfate solid is white. Pour copper (ii) sulfate solution into. Use this demonstration to find the value of the faraday. Copper Ii Sulfate Electrolysis Products.
From www.youtube.com
ELECTROLYSIS OF COPPER SULPHATE (CuSO4) YouTube Copper Ii Sulfate Electrolysis Products Pour copper (ii) sulfate solution into. Electrolysis of copper sulfate (cuso 4) solution. Use this demonstration to find the value of the faraday constant from electrolysis of copper (ii) sulfate solution. Electrolysis of copper (ii) sulfate using graphite electrodes. Using the simple apparatus (above left diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the. Copper Ii Sulfate Electrolysis Products.
From giolacmfh.blob.core.windows.net
Copper Sulfate Conductivity at Ryan Wiley blog Copper Ii Sulfate Electrolysis Products Electrolysis of copper (ii) sulfate using graphite electrodes. Electrolysis of copper sulfate (cuso 4) solution. Copper sulfate is soluble in water and gives a blue colour aqueous solution though anhydrous copper sulfate solid is white. In this experiment, students learn how the value of the faraday. Explore the electrolysis of copper(ii) sulfate solution and related industrial processes with this class. Copper Ii Sulfate Electrolysis Products.
From socratic.org
What happens on the cathode during the electrolysis of a copper(II Copper Ii Sulfate Electrolysis Products This experiment is designed to demonstrate the different products obtained when the electrolysis of copper(ii) sulfate solution is carried out first. Using the simple apparatus (above left diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis of copper sulfate solution are (i) a. Electrolysis of copper (ii) sulfate using graphite electrodes. Pour copper. Copper Ii Sulfate Electrolysis Products.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Copper Ii Sulfate Electrolysis Products This experiment is designed to demonstrate the different products obtained when the electrolysis of copper(ii) sulfate solution is carried out first. Electrolysis involves using electricity to break. Using the simple apparatus (above left diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis of copper sulfate solution are (i) a. Copper sulfate is soluble. Copper Ii Sulfate Electrolysis Products.
From byjus.com
With the help of a diagram explain the method of refining of copper by Copper Ii Sulfate Electrolysis Products Electrolysis involves using electricity to break. Electrolysis of copper sulfate (cuso 4) solution. This experiment is designed to demonstrate the different products obtained when the electrolysis of copper(ii) sulfate solution is carried out first. Includes kit list and safety instructions. Electrolysis of copper (ii) sulfate using graphite electrodes. Explore the electrolysis of copper(ii) sulfate solution and related industrial processes with. Copper Ii Sulfate Electrolysis Products.
From nationaldefensepac.org
Principle Of Electrolysis Of Copper Sulfate Electrolyte, 57 OFF Copper Ii Sulfate Electrolysis Products Pour copper (ii) sulfate solution into. Use this demonstration to find the value of the faraday constant from electrolysis of copper (ii) sulfate solution. Copper sulfate is soluble in water and gives a blue colour aqueous solution though anhydrous copper sulfate solid is white. There are different observations for each electrode. This experiment is designed to demonstrate the different products. Copper Ii Sulfate Electrolysis Products.
From www.youtube.com
Electrolysis of Copper Sulphate YouTube Copper Ii Sulfate Electrolysis Products Use this demonstration to find the value of the faraday constant from electrolysis of copper (ii) sulfate solution. Electrolysis of copper (ii) sulfate using graphite electrodes. Explore the electrolysis of copper(ii) sulfate solution and related industrial processes with this class experiment. In this experiment, students learn how the value of the faraday. Includes kit list and safety instructions. Electrolysis of. Copper Ii Sulfate Electrolysis Products.
From www.vecteezy.com
Electrolysis of copper sulfate solution with impure copper anode and Copper Ii Sulfate Electrolysis Products This experiment is designed to demonstrate the different products obtained when the electrolysis of copper(ii) sulfate solution is carried out first. Pour copper (ii) sulfate solution into. Electrolysis involves using electricity to break. There are different observations for each electrode. Explore the electrolysis of copper(ii) sulfate solution and related industrial processes with this class experiment. Electrolysis of copper (ii) sulfate. Copper Ii Sulfate Electrolysis Products.
From in.pinterest.com
Advanced Chemistry in Creation 2nd Edition Student Text Copper Ii Sulfate Electrolysis Products Electrolysis of copper sulfate (cuso 4) solution. Includes kit list and safety instructions. Explore the electrolysis of copper(ii) sulfate solution and related industrial processes with this class experiment. Electrolysis involves using electricity to break. Using the simple apparatus (above left diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis of copper sulfate solution. Copper Ii Sulfate Electrolysis Products.
From edu.rsc.org
Electrolysis of copper(II) sulfate solution Experiment RSC Education Copper Ii Sulfate Electrolysis Products Use this demonstration to find the value of the faraday constant from electrolysis of copper (ii) sulfate solution. Copper sulfate is soluble in water and gives a blue colour aqueous solution though anhydrous copper sulfate solid is white. Includes kit list and safety instructions. Using the simple apparatus (above left diagram) and inert carbon (graphite) or platinum electrodes, you can. Copper Ii Sulfate Electrolysis Products.
From www.alamy.com
Electrolysis of copper sulphate solution using copper electrodes Stock Copper Ii Sulfate Electrolysis Products Electrolysis of copper sulfate (cuso 4) solution. Electrolysis of copper (ii) sulfate using graphite electrodes. In this experiment, students learn how the value of the faraday. Pour copper (ii) sulfate solution into. Use this demonstration to find the value of the faraday constant from electrolysis of copper (ii) sulfate solution. Copper sulfate is soluble in water and gives a blue. Copper Ii Sulfate Electrolysis Products.
From www.youtube.com
Electrolysis of Copper Sulphate for making Sulphuric Acid YouTube Copper Ii Sulfate Electrolysis Products In this experiment, students learn how the value of the faraday. Includes kit list and safety instructions. Use this demonstration to find the value of the faraday constant from electrolysis of copper (ii) sulfate solution. Electrolysis of copper sulfate (cuso 4) solution. Copper sulfate is soluble in water and gives a blue colour aqueous solution though anhydrous copper sulfate solid. Copper Ii Sulfate Electrolysis Products.
From electro-lysis.blogspot.com
Electro electricity lysisbreak down January 2011 Copper Ii Sulfate Electrolysis Products Electrolysis involves using electricity to break. Electrolysis of copper (ii) sulfate using graphite electrodes. Pour copper (ii) sulfate solution into. There are different observations for each electrode. This experiment is designed to demonstrate the different products obtained when the electrolysis of copper(ii) sulfate solution is carried out first. In this experiment, students learn how the value of the faraday. Electrolysis. Copper Ii Sulfate Electrolysis Products.
From www.doubtnut.com
Copper sulphate solution is electrolysed using copper electrodes. Copper Ii Sulfate Electrolysis Products Electrolysis of copper (ii) sulfate using graphite electrodes. Explore the electrolysis of copper(ii) sulfate solution and related industrial processes with this class experiment. Electrolysis of copper sulfate (cuso 4) solution. Using the simple apparatus (above left diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis of copper sulfate solution are (i) a. Includes. Copper Ii Sulfate Electrolysis Products.
From spmscience.blog.onlinetuition.com.my
5.5.2 Electrolysis of Copper (II) Chloride Solution SPM Science Copper Ii Sulfate Electrolysis Products In this experiment, students learn how the value of the faraday. Electrolysis involves using electricity to break. This experiment is designed to demonstrate the different products obtained when the electrolysis of copper(ii) sulfate solution is carried out first. Includes kit list and safety instructions. Electrolysis of copper (ii) sulfate using graphite electrodes. Pour copper (ii) sulfate solution into. Explore the. Copper Ii Sulfate Electrolysis Products.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS Copper Ii Sulfate Electrolysis Products Includes kit list and safety instructions. Electrolysis of copper (ii) sulfate using graphite electrodes. Pour copper (ii) sulfate solution into. Explore the electrolysis of copper(ii) sulfate solution and related industrial processes with this class experiment. Use this demonstration to find the value of the faraday constant from electrolysis of copper (ii) sulfate solution. In this experiment, students learn how the. Copper Ii Sulfate Electrolysis Products.
From informacionpublica.svet.gob.gt
ELECTROLYSIS OF COPPER SULPHATE (CuSO4) Copper Ii Sulfate Electrolysis Products Electrolysis of copper sulfate (cuso 4) solution. There are different observations for each electrode. Electrolysis of copper (ii) sulfate using graphite electrodes. Use this demonstration to find the value of the faraday constant from electrolysis of copper (ii) sulfate solution. Electrolysis involves using electricity to break. Explore the electrolysis of copper(ii) sulfate solution and related industrial processes with this class. Copper Ii Sulfate Electrolysis Products.
From byjus.com
Explain the electrolysis of copper sulphate solution with the help of a Copper Ii Sulfate Electrolysis Products Electrolysis of copper (ii) sulfate using graphite electrodes. Pour copper (ii) sulfate solution into. This experiment is designed to demonstrate the different products obtained when the electrolysis of copper(ii) sulfate solution is carried out first. Explore the electrolysis of copper(ii) sulfate solution and related industrial processes with this class experiment. There are different observations for each electrode. Use this demonstration. Copper Ii Sulfate Electrolysis Products.
From chem2u.blogspot.my
chem2U November 2013 Copper Ii Sulfate Electrolysis Products This experiment is designed to demonstrate the different products obtained when the electrolysis of copper(ii) sulfate solution is carried out first. Includes kit list and safety instructions. Electrolysis of copper (ii) sulfate using graphite electrodes. Explore the electrolysis of copper(ii) sulfate solution and related industrial processes with this class experiment. Copper sulfate is soluble in water and gives a blue. Copper Ii Sulfate Electrolysis Products.