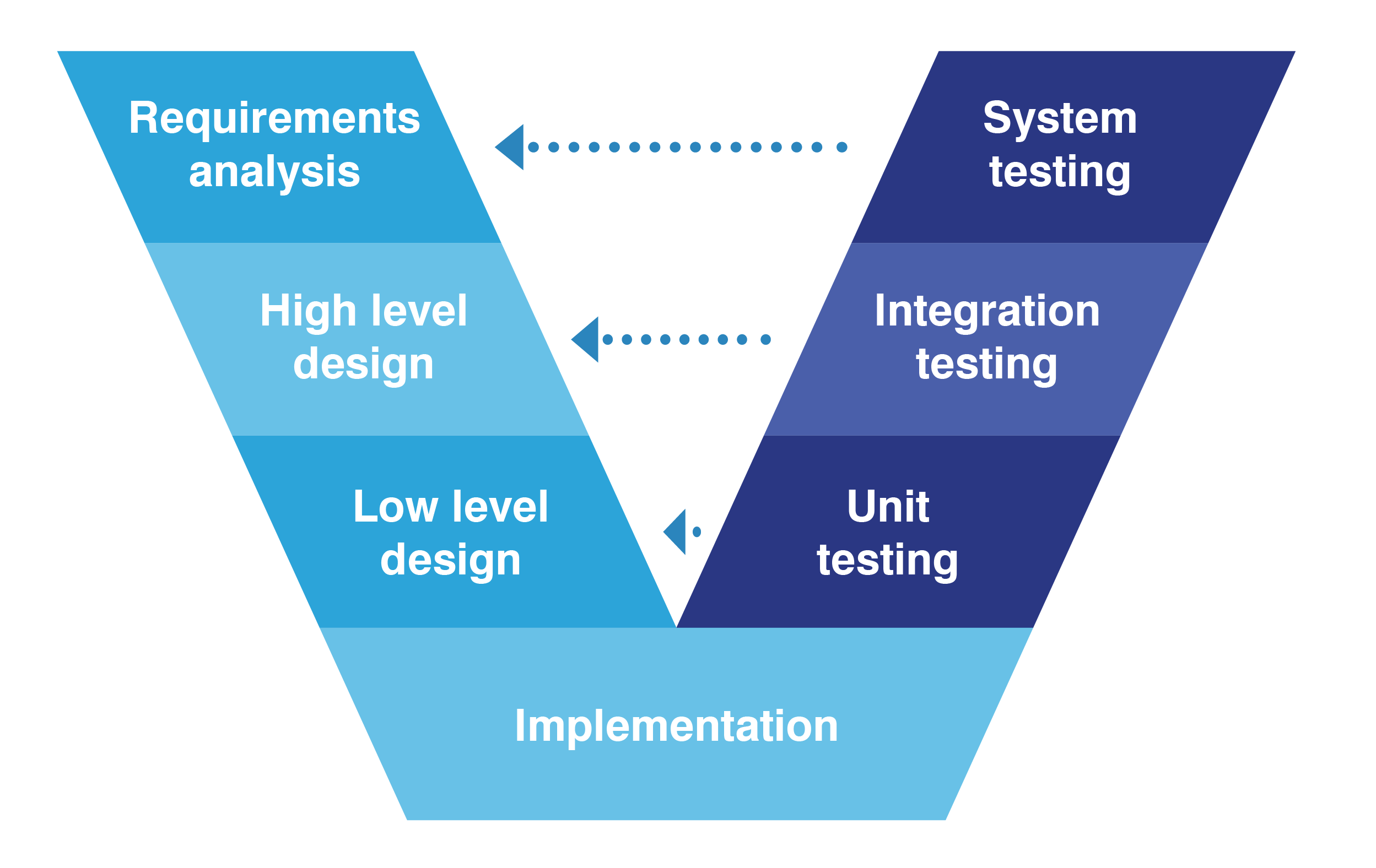
V Model Equipment Qualification . The universal “v model” approach shall be followed for all the qualification and. As defined and standardized in the second edition of the commissioning. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. Do you find it difficult to decide whether to qualify or. It is further divided into dq, iq, oq & pq.
from www.rapitasystems.com
The universal “v model” approach shall be followed for all the qualification and. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. It is further divided into dq, iq, oq & pq. Do you find it difficult to decide whether to qualify or. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated. As defined and standardized in the second edition of the commissioning.
Certifying eVTOL Rapita Systems
V Model Equipment Qualification The universal “v model” approach shall be followed for all the qualification and. Do you find it difficult to decide whether to qualify or. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated. It is further divided into dq, iq, oq & pq. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. The universal “v model” approach shall be followed for all the qualification and. As defined and standardized in the second edition of the commissioning.
From www.softwaretestingmaterial.com
V Model in Software Development Life Cycle V Model Equipment Qualification It is further divided into dq, iq, oq & pq. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. As defined and standardized in the second edition of the commissioning. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated.. V Model Equipment Qualification.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and V Model Equipment Qualification It is further divided into dq, iq, oq & pq. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated. As defined and standardized in the second edition of the commissioning. Development models is the “v” model, which is a framework or structure for undertaking the design,. V Model Equipment Qualification.
From www.qv-compliance.dk
Qualification and Validation V Model Equipment Qualification Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. As defined and standardized in the second edition of the commissioning. The universal “v model” approach shall be followed for all the. V Model Equipment Qualification.
From www.rotronic.com
Validation / Qualification ROTRONIC Measurement Solutions V Model Equipment Qualification The universal “v model” approach shall be followed for all the qualification and. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. As defined and standardized in the second edition of the commissioning. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities,. V Model Equipment Qualification.
From www.nalys-group.com
V cycle in the pharmaceutical environment Nalys V Model Equipment Qualification It is further divided into dq, iq, oq & pq. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated. The universal “v model” approach shall be followed for all the qualification and. Development models is the “v” model, which is a framework or structure for undertaking. V Model Equipment Qualification.
From www.kewaunee.in
The VModel Approach to Lab Validation Explained Kewaunee V Model Equipment Qualification The universal “v model” approach shall be followed for all the qualification and. It is further divided into dq, iq, oq & pq. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. As defined and standardized in the second edition of the commissioning. Qualification is documented evidence. V Model Equipment Qualification.
From pharmaanalytic.com
Equipment Qualification V Model Equipment Qualification It is further divided into dq, iq, oq & pq. Do you find it difficult to decide whether to qualify or. The universal “v model” approach shall be followed for all the qualification and. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. As defined and standardized in the second edition of the. V Model Equipment Qualification.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and V Model Equipment Qualification Do you find it difficult to decide whether to qualify or. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated. Development models is the “v” model, which is a framework. V Model Equipment Qualification.
From www.presentationeze.com
Equipment Validation Facility Qualification Material V Model Equipment Qualification As defined and standardized in the second edition of the commissioning. The universal “v model” approach shall be followed for all the qualification and. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. It is further divided into dq, iq, oq & pq. Do you find it difficult to decide whether to qualify. V Model Equipment Qualification.
From www.slideserve.com
PPT EQUIPMENT AND ITS QUALIFICATION PowerPoint Presentation, free V Model Equipment Qualification The universal “v model” approach shall be followed for all the qualification and. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. Do you find it difficult to decide whether to. V Model Equipment Qualification.
From insights.sei.cmu.edu
Using V Models for Testing V Model Equipment Qualification As defined and standardized in the second edition of the commissioning. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. Do you find it difficult to decide whether to qualify or. The purpose of this updated guidance is to define the baseline® approach for the commissioning and. V Model Equipment Qualification.
From www.vrogue.co
Equipment Qualification Procedure And Protocol Guidel vrogue.co V Model Equipment Qualification The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. It is further divided into dq, iq, oq & pq. Do you find it difficult to decide whether to qualify or.. V Model Equipment Qualification.
From www.researchgate.net
4 VModel for Direct Impact Systems Download Scientific Diagram V Model Equipment Qualification Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. As defined and standardized in the second edition of the commissioning. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. It is further divided into dq, iq, oq & pq. The. V Model Equipment Qualification.
From www.rapitasystems.com
Certifying eVTOL Rapita Systems V Model Equipment Qualification Do you find it difficult to decide whether to qualify or. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated. It is further divided into dq, iq, oq & pq. As defined and standardized in the second edition of the commissioning. The universal “v model” approach. V Model Equipment Qualification.
From qbdgroup.com
What is the GAMP 5 Vmodel in CSV? QbD Group V Model Equipment Qualification Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. The universal “v model” approach shall be followed for all the qualification and. It is further divided into dq, iq, oq & pq. Do you find it difficult to decide whether to qualify or. Development models is the “v” model, which is a framework. V Model Equipment Qualification.
From pharmatreasures.blogspot.com
Pharma Treasures V Model Validation Concept in Pharmaceuticals V Model Equipment Qualification Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated. It is further divided into dq, iq, oq & pq. Do you find it difficult to decide whether to qualify or.. V Model Equipment Qualification.
From testingbuddy.co.in
Vmodel/ V and V model /Verification and Validation model Testingbuddy V Model Equipment Qualification Do you find it difficult to decide whether to qualify or. It is further divided into dq, iq, oq & pq. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. As defined and standardized in the second edition of the commissioning. The purpose of this updated guidance. V Model Equipment Qualification.
From www.pinterest.com.mx
Six Sigma Validation Process IQ Installation Qualification OQ V Model Equipment Qualification It is further divided into dq, iq, oq & pq. As defined and standardized in the second edition of the commissioning. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. Do you find it difficult to decide whether to qualify or. The purpose of this updated guidance is to define the baseline® approach. V Model Equipment Qualification.
From tutorialshut.com
What is V Model in SDLC (Verification and Validation Model) Tutorials Hut V Model Equipment Qualification Do you find it difficult to decide whether to qualify or. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. It is further divided into dq, iq, oq & pq. The. V Model Equipment Qualification.
From qualsys-1.hubspotpagebuilder.com
3 powerful quality methodologies you should be applying V Model Equipment Qualification The universal “v model” approach shall be followed for all the qualification and. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated. As defined. V Model Equipment Qualification.
From www.pcfarmacia.in
GAMP 5 Vmodel in CSV V Model Equipment Qualification It is further divided into dq, iq, oq & pq. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. Do you find it difficult to decide whether to qualify or. As defined and standardized in the second edition of the commissioning. The purpose of this updated guidance. V Model Equipment Qualification.
From www.cognidox.com
A guide to GXP compliance V Model Equipment Qualification Do you find it difficult to decide whether to qualify or. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. The universal “v model” approach shall be followed for all the. V Model Equipment Qualification.
From philipbroxtonbrown.wordpress.com
301 Moved Permanently V Model Equipment Qualification The universal “v model” approach shall be followed for all the qualification and. As defined and standardized in the second edition of the commissioning. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities,. V Model Equipment Qualification.
From www.cytivalifesciences.com
Stay compliant instrument qualification for cGMP Cytiva V Model Equipment Qualification It is further divided into dq, iq, oq & pq. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. Do you find it difficult to decide whether to qualify or. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated.. V Model Equipment Qualification.
From www.programsbuzz.com
Software Development and Software Testing V Model Equipment Qualification Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. It is further divided into dq, iq, oq & pq. Do you find it difficult to decide whether to qualify or. As. V Model Equipment Qualification.
From www.hamiltoncompany.com
Operation Qualification & Installation Qualification Hamilton Company V Model Equipment Qualification As defined and standardized in the second edition of the commissioning. The universal “v model” approach shall be followed for all the qualification and. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. The purpose of this updated guidance is to define the baseline® approach for the. V Model Equipment Qualification.
From journals.sagepub.com
Qualification of Robotic Laboratory Equipment Christine Caillet, Yves V Model Equipment Qualification The universal “v model” approach shall be followed for all the qualification and. Do you find it difficult to decide whether to qualify or. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated. It is further divided into dq, iq, oq & pq. As defined and. V Model Equipment Qualification.
From www.getreskilled.com
What are IQ OQ PQ? Why are they Critical to the Pharma Industry? V Model Equipment Qualification The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. It is further divided into dq, iq, oq & pq. Do you find it difficult. V Model Equipment Qualification.
From melsatar.blog
The Validation and Verification Model The VModel Mohamed Sami V Model Equipment Qualification As defined and standardized in the second edition of the commissioning. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. Development models is the “v” model, which is a framework. V Model Equipment Qualification.
From www.slideteam.net
Itil service v model of validation and testing Presentation Graphics V Model Equipment Qualification Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. It is further divided into dq, iq, oq & pq. As defined and standardized in the second edition of the commissioning. Do you find it difficult to decide whether to qualify or. Qualification is documented evidence that a. V Model Equipment Qualification.
From tobmanagement.nl
Asset Life Cycle & Validation V Model Equipment Qualification It is further divided into dq, iq, oq & pq. Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated. The universal “v model” approach shall be followed for all the. V Model Equipment Qualification.
From farmasiindustri.com
Pentingnya Validasi dalam Industri Farmasi FARMASI INDUSTRI V Model Equipment Qualification Qualification is documented evidence that a specific equipment, facility or system is fit/ready for intended use. The universal “v model” approach shall be followed for all the qualification and. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. It is further divided into dq, iq, oq &. V Model Equipment Qualification.
From www.youtube.com
Equipment Validation in Pharmaceutical Industry DQ IQ OQ PQ YouTube V Model Equipment Qualification Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. It is further divided into dq, iq, oq & pq. The purpose of this updated guidance is to define the baseline® approach for the commissioning and qualification of facilities, utilities, and equipment regulated. Qualification is documented evidence that. V Model Equipment Qualification.
From testingmaniac007.blogspot.com
V MODEL / V & V MODEL (Verification and Validation Model ) Software V Model Equipment Qualification Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. As defined and standardized in the second edition of the commissioning. Do you find it difficult to decide whether to qualify or. The universal “v model” approach shall be followed for all the qualification and. It is further. V Model Equipment Qualification.
From www.vrogue.co
Equipment Qualification Procedure And Protocol Guidel vrogue.co V Model Equipment Qualification The universal “v model” approach shall be followed for all the qualification and. Development models is the “v” model, which is a framework or structure for undertaking the design, execution, commissioning and qualification of a. As defined and standardized in the second edition of the commissioning. It is further divided into dq, iq, oq & pq. Do you find it. V Model Equipment Qualification.