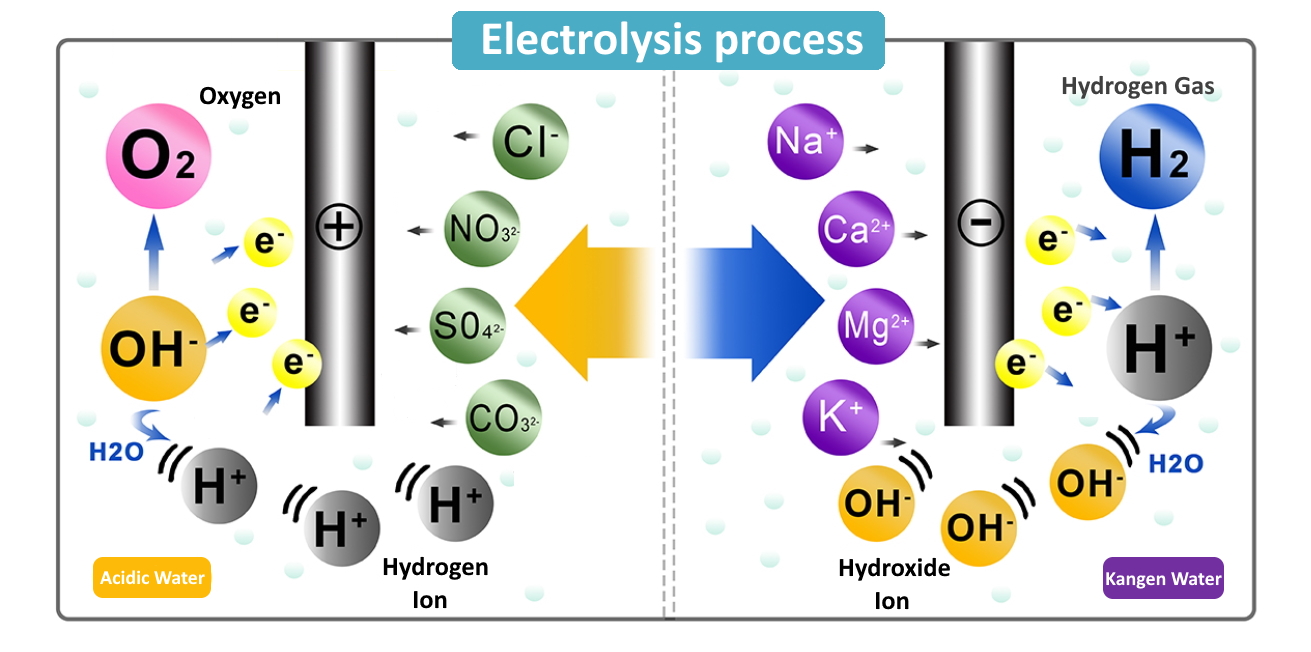
What Happens At The Electrodes During Electrolysis . Reactive metals are extracted from their ores using electrolysis. This separates the ions, and elements will be discharged at the electrodes. At the anode, water is oxidized to oxygen gas and hydrogen ions. The products of electrolysis can be predicted for a given. The positive electrode, on the other hand, will attract negative ions (anions) toward itself. Inert electrodes should be used, otherwise they will react with the solution/products. Ionic compounds conduct electricity when molten or in solution. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Electrolysis involves using electricity to break down electrolytes to form elements. Reactive metals are extracted from their ores using electrolysis. At the cathode, water is reduced to hydrogen gas and hydroxide. Electrolysis is the process of passing electrical current (direct current) through a solution or molten ionic compound, to decompose electrolytes. Ionic compounds conduct electricity when molten or in solution. When we perform electrolysis, we use two electrodes. One electrode is positively charged, which is called the anode.
from www.enagic-asia.com
This separates the ions, and elements will be discharged at the electrodes. Ionic compounds conduct electricity when molten or in solution. When we perform electrolysis, we use two electrodes. The products of electrolysis can be predicted for a given. At the anode, water is oxidized to oxygen gas and hydrogen ions. Reactive metals are extracted from their ores using electrolysis. At the cathode, water is reduced to hydrogen gas and hydroxide. Reactive metals are extracted from their ores using electrolysis. Electrolysis involves using electricity to break down electrolytes to form elements. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode.
The electrolysis process Enagic Kangen Water
What Happens At The Electrodes During Electrolysis The positive electrode, on the other hand, will attract negative ions (anions) toward itself. At the cathode, water is reduced to hydrogen gas and hydroxide. Electrolysis involves using electricity to break down electrolytes to form elements. Reactive metals are extracted from their ores using electrolysis. Electrolysis is the process of passing electrical current (direct current) through a solution or molten ionic compound, to decompose electrolytes. The products of electrolysis can be predicted for a given. Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. At the anode, water is oxidized to oxygen gas and hydrogen ions. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Inert electrodes should be used, otherwise they will react with the solution/products. One electrode is positively charged, which is called the anode. This separates the ions, and elements will be discharged at the electrodes. Ionic compounds conduct electricity when molten or in solution. When we perform electrolysis, we use two electrodes. The positive electrode, on the other hand, will attract negative ions (anions) toward itself.
From www.knowledgeboat.com
Chapter 6 Electrolysis Selina Solutions Concise Chemistry Class 10 What Happens At The Electrodes During Electrolysis Ionic compounds conduct electricity when molten or in solution. Reactive metals are extracted from their ores using electrolysis. At the anode, water is oxidized to oxygen gas and hydrogen ions. Electrolysis is the process of passing electrical current (direct current) through a solution or molten ionic compound, to decompose electrolytes. Inert electrodes should be used, otherwise they will react with. What Happens At The Electrodes During Electrolysis.
From owlcation.com
Electrolysis The Way of the Future Owlcation What Happens At The Electrodes During Electrolysis Electrolysis involves using electricity to break down electrolytes to form elements. Reactive metals are extracted from their ores using electrolysis. At the anode, water is oxidized to oxygen gas and hydrogen ions. At the cathode, water is reduced to hydrogen gas and hydroxide. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode.. What Happens At The Electrodes During Electrolysis.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts What Happens At The Electrodes During Electrolysis At the anode, water is oxidized to oxygen gas and hydrogen ions. The positive electrode, on the other hand, will attract negative ions (anions) toward itself. One electrode is positively charged, which is called the anode. Inert electrodes should be used, otherwise they will react with the solution/products. When we perform electrolysis, we use two electrodes. Electrolysis involves using electricity. What Happens At The Electrodes During Electrolysis.
From courses.lumenlearning.com
Predicting the Products of Electrolysis Introduction to Chemistry What Happens At The Electrodes During Electrolysis At the cathode, water is reduced to hydrogen gas and hydroxide. The positive electrode, on the other hand, will attract negative ions (anions) toward itself. Reactive metals are extracted from their ores using electrolysis. At the anode, water is oxidized to oxygen gas and hydrogen ions. Electrolysis is the process of passing electrical current (direct current) through a solution or. What Happens At The Electrodes During Electrolysis.
From socratic.org
What is electrolysis? Socratic What Happens At The Electrodes During Electrolysis At the anode, water is oxidized to oxygen gas and hydrogen ions. This separates the ions, and elements will be discharged at the electrodes. Ionic compounds conduct electricity when molten or in solution. Electrolysis involves using electricity to break down electrolytes to form elements. At the cathode, water is reduced to hydrogen gas and hydroxide. Inert electrodes should be used,. What Happens At The Electrodes During Electrolysis.
From chemistry.stackexchange.com
chemistry What is the greenish substance formed after the What Happens At The Electrodes During Electrolysis Ionic compounds conduct electricity when molten or in solution. Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given. At the anode, water is oxidized to oxygen gas and hydrogen ions. Inert electrodes should be used, otherwise they will react with the solution/products. When we perform electrolysis, we use. What Happens At The Electrodes During Electrolysis.
From www.enagic-asia.com
The electrolysis process Enagic Kangen Water What Happens At The Electrodes During Electrolysis Electrolysis involves using electricity to break down electrolytes to form elements. The positive electrode, on the other hand, will attract negative ions (anions) toward itself. Ionic compounds conduct electricity when molten or in solution. One electrode is positively charged, which is called the anode. Ionic compounds conduct electricity when molten or in solution. This separates the ions, and elements will. What Happens At The Electrodes During Electrolysis.
From chem.libretexts.org
Electrolysis I Chemistry LibreTexts What Happens At The Electrodes During Electrolysis When we perform electrolysis, we use two electrodes. This separates the ions, and elements will be discharged at the electrodes. At the cathode, water is reduced to hydrogen gas and hydroxide. Ionic compounds conduct electricity when molten or in solution. Electrolysis is the process of passing electrical current (direct current) through a solution or molten ionic compound, to decompose electrolytes.. What Happens At The Electrodes During Electrolysis.
From www.nagwa.com
Question Video Recalling the Name of the Positive Electrode in an What Happens At The Electrodes During Electrolysis When we perform electrolysis, we use two electrodes. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. At the cathode, water is reduced to hydrogen gas and hydroxide. Ionic compounds conduct electricity when molten or in solution. Electrolysis is the process of passing electrical current (direct current) through a solution or molten. What Happens At The Electrodes During Electrolysis.
From www.vecteezy.com
Electrolysis of copper sulfate solution with impure copper anode and What Happens At The Electrodes During Electrolysis When we perform electrolysis, we use two electrodes. Electrolysis involves using electricity to break down electrolytes to form elements. The positive electrode, on the other hand, will attract negative ions (anions) toward itself. The products of electrolysis can be predicted for a given. One electrode is positively charged, which is called the anode. Electrolysis is the process of passing electrical. What Happens At The Electrodes During Electrolysis.
From www.youtube.com
Electrolysis of dilute sodium chloride (inert electrodes) YouTube What Happens At The Electrodes During Electrolysis Reactive metals are extracted from their ores using electrolysis. The products of electrolysis can be predicted for a given. When we perform electrolysis, we use two electrodes. At the cathode, water is reduced to hydrogen gas and hydroxide. The positive electrode, on the other hand, will attract negative ions (anions) toward itself. Electrolysis involves using electricity to break down electrolytes. What Happens At The Electrodes During Electrolysis.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis What Happens At The Electrodes During Electrolysis At the cathode, water is reduced to hydrogen gas and hydroxide. Electrolysis involves using electricity to break down electrolytes to form elements. When we perform electrolysis, we use two electrodes. One electrode is positively charged, which is called the anode. Inert electrodes should be used, otherwise they will react with the solution/products. This separates the ions, and elements will be. What Happens At The Electrodes During Electrolysis.
From www.coursehero.com
Electrolysis Chemistry for Majors Atoms First Course Hero What Happens At The Electrodes During Electrolysis This separates the ions, and elements will be discharged at the electrodes. At the anode, water is oxidized to oxygen gas and hydrogen ions. Electrolysis involves using electricity to break down electrolytes to form elements. Reactive metals are extracted from their ores using electrolysis. Reactive metals are extracted from their ores using electrolysis. Electrolysis is the process of passing electrical. What Happens At The Electrodes During Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk What Happens At The Electrodes During Electrolysis Reactive metals are extracted from their ores using electrolysis. When we perform electrolysis, we use two electrodes. Electrolysis involves using electricity to break down electrolytes to form elements. One electrode is positively charged, which is called the anode. Inert electrodes should be used, otherwise they will react with the solution/products. Reactive metals are extracted from their ores using electrolysis. This. What Happens At The Electrodes During Electrolysis.
From enginelibsaprozoic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis What Happens At The Electrodes During Electrolysis When we perform electrolysis, we use two electrodes. Ionic compounds conduct electricity when molten or in solution. At the anode, water is oxidized to oxygen gas and hydrogen ions. The products of electrolysis can be predicted for a given. Ionic compounds conduct electricity when molten or in solution. This separates the ions, and elements will be discharged at the electrodes.. What Happens At The Electrodes During Electrolysis.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.58C Describe Experiments to Investigate What Happens At The Electrodes During Electrolysis In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. At the anode, water is oxidized to oxygen gas and hydrogen ions. Reactive metals are extracted from their ores using electrolysis. The positive electrode, on the other hand, will attract negative ions (anions) toward itself. Reactive metals are extracted from their ores using. What Happens At The Electrodes During Electrolysis.
From giowrvapv.blob.core.windows.net
What Electrodes Are Used In Electrolysis at Patricia Nutt blog What Happens At The Electrodes During Electrolysis Ionic compounds conduct electricity when molten or in solution. Ionic compounds conduct electricity when molten or in solution. The products of electrolysis can be predicted for a given. At the cathode, water is reduced to hydrogen gas and hydroxide. Reactive metals are extracted from their ores using electrolysis. Reactive metals are extracted from their ores using electrolysis. One electrode is. What Happens At The Electrodes During Electrolysis.
From morrisclassicalacademy.blogspot.com
Morris Classical Academy Electrolysis of water What Happens At The Electrodes During Electrolysis This separates the ions, and elements will be discharged at the electrodes. One electrode is positively charged, which is called the anode. At the anode, water is oxidized to oxygen gas and hydrogen ions. Ionic compounds conduct electricity when molten or in solution. The products of electrolysis can be predicted for a given. Ionic compounds conduct electricity when molten or. What Happens At The Electrodes During Electrolysis.
From www.onlinemathlearning.com
Chemical Reactions IGCSE Chemistry (solutions, examples, worksheets What Happens At The Electrodes During Electrolysis The positive electrode, on the other hand, will attract negative ions (anions) toward itself. Reactive metals are extracted from their ores using electrolysis. Reactive metals are extracted from their ores using electrolysis. At the anode, water is oxidized to oxygen gas and hydrogen ions. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. What Happens At The Electrodes During Electrolysis.
From mungfali.com
Electrolysis Process Diagram What Happens At The Electrodes During Electrolysis One electrode is positively charged, which is called the anode. Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. At the anode, water is oxidized to oxygen gas and hydrogen ions. Electrolysis is the process of passing electrical current (direct current) through a solution or molten ionic compound, to decompose electrolytes.. What Happens At The Electrodes During Electrolysis.
From www.snexplores.org
Explainer What is an electrode? What Happens At The Electrodes During Electrolysis Ionic compounds conduct electricity when molten or in solution. Reactive metals are extracted from their ores using electrolysis. At the anode, water is oxidized to oxygen gas and hydrogen ions. The positive electrode, on the other hand, will attract negative ions (anions) toward itself. Ionic compounds conduct electricity when molten or in solution. In any electrochemical cell (electrolytic or galvanic). What Happens At The Electrodes During Electrolysis.
From study.com
Electrolysis of Aqueous Solutions Lesson What Happens At The Electrodes During Electrolysis This separates the ions, and elements will be discharged at the electrodes. One electrode is positively charged, which is called the anode. Electrolysis involves using electricity to break down electrolytes to form elements. Inert electrodes should be used, otherwise they will react with the solution/products. Ionic compounds conduct electricity when molten or in solution. Reactive metals are extracted from their. What Happens At The Electrodes During Electrolysis.
From chem2u.blogspot.com
chem2U Electrolysis of Copper(II) Sulphate What Happens At The Electrodes During Electrolysis At the anode, water is oxidized to oxygen gas and hydrogen ions. Electrolysis is the process of passing electrical current (direct current) through a solution or molten ionic compound, to decompose electrolytes. When we perform electrolysis, we use two electrodes. Reactive metals are extracted from their ores using electrolysis. Inert electrodes should be used, otherwise they will react with the. What Happens At The Electrodes During Electrolysis.
From resource.studiaacademy.com
1.9 Electrolysis Studia Academy Resources What Happens At The Electrodes During Electrolysis At the anode, water is oxidized to oxygen gas and hydrogen ions. At the cathode, water is reduced to hydrogen gas and hydroxide. Ionic compounds conduct electricity when molten or in solution. Reactive metals are extracted from their ores using electrolysis. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. This separates. What Happens At The Electrodes During Electrolysis.
From circuitcoffeegirl89t1.z14.web.core.windows.net
What Forms At The Cathode During Electrolysis What Happens At The Electrodes During Electrolysis In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. At the cathode, water is reduced to hydrogen gas and hydroxide. This separates the ions, and elements will be discharged at the electrodes. One electrode is positively charged, which is called the anode. At the anode, water is oxidized to oxygen gas and. What Happens At The Electrodes During Electrolysis.
From webmis.highland.cc.il.us
Electrolysis What Happens At The Electrodes During Electrolysis Reactive metals are extracted from their ores using electrolysis. Reactive metals are extracted from their ores using electrolysis. Inert electrodes should be used, otherwise they will react with the solution/products. At the cathode, water is reduced to hydrogen gas and hydroxide. When we perform electrolysis, we use two electrodes. Ionic compounds conduct electricity when molten or in solution. This separates. What Happens At The Electrodes During Electrolysis.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte What Happens At The Electrodes During Electrolysis When we perform electrolysis, we use two electrodes. This separates the ions, and elements will be discharged at the electrodes. The positive electrode, on the other hand, will attract negative ions (anions) toward itself. Ionic compounds conduct electricity when molten or in solution. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode.. What Happens At The Electrodes During Electrolysis.
From mungfali.com
Electrolysis Process Diagram What Happens At The Electrodes During Electrolysis The products of electrolysis can be predicted for a given. The positive electrode, on the other hand, will attract negative ions (anions) toward itself. Reactive metals are extracted from their ores using electrolysis. Electrolysis involves using electricity to break down electrolytes to form elements. At the anode, water is oxidized to oxygen gas and hydrogen ions. This separates the ions,. What Happens At The Electrodes During Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk What Happens At The Electrodes During Electrolysis When we perform electrolysis, we use two electrodes. Ionic compounds conduct electricity when molten or in solution. The positive electrode, on the other hand, will attract negative ions (anions) toward itself. Reactive metals are extracted from their ores using electrolysis. At the anode, water is oxidized to oxygen gas and hydrogen ions. Inert electrodes should be used, otherwise they will. What Happens At The Electrodes During Electrolysis.
From chem.libretexts.org
2.2 Standard Electrode Potentials Chemistry LibreTexts What Happens At The Electrodes During Electrolysis The positive electrode, on the other hand, will attract negative ions (anions) toward itself. Ionic compounds conduct electricity when molten or in solution. At the anode, water is oxidized to oxygen gas and hydrogen ions. Electrolysis is the process of passing electrical current (direct current) through a solution or molten ionic compound, to decompose electrolytes. At the cathode, water is. What Happens At The Electrodes During Electrolysis.
From www.youtube.com
WATER ELECTROLYSIS DEMONSTRATION WITH EXPLANATION CHEMISTRY GRADE 8 What Happens At The Electrodes During Electrolysis Reactive metals are extracted from their ores using electrolysis. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The positive electrode, on the other hand, will attract negative ions (anions) toward itself. Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. Electrolysis involves. What Happens At The Electrodes During Electrolysis.
From www.oceangeothermal.org
Hydrogen Through Electrolysis Ocean Geothermal Energy Foundation What Happens At The Electrodes During Electrolysis This separates the ions, and elements will be discharged at the electrodes. Ionic compounds conduct electricity when molten or in solution. At the cathode, water is reduced to hydrogen gas and hydroxide. Electrolysis involves using electricity to break down electrolytes to form elements. The positive electrode, on the other hand, will attract negative ions (anions) toward itself. At the anode,. What Happens At The Electrodes During Electrolysis.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps What Happens At The Electrodes During Electrolysis Reactive metals are extracted from their ores using electrolysis. The products of electrolysis can be predicted for a given. Ionic compounds conduct electricity when molten or in solution. Reactive metals are extracted from their ores using electrolysis. Electrolysis involves using electricity to break down electrolytes to form elements. Ionic compounds conduct electricity when molten or in solution. Inert electrodes should. What Happens At The Electrodes During Electrolysis.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download What Happens At The Electrodes During Electrolysis The products of electrolysis can be predicted for a given. This separates the ions, and elements will be discharged at the electrodes. At the anode, water is oxidized to oxygen gas and hydrogen ions. One electrode is positively charged, which is called the anode. When we perform electrolysis, we use two electrodes. Electrolysis involves using electricity to break down electrolytes. What Happens At The Electrodes During Electrolysis.
From byjus.com
Electrolysis of cuso4 solution using copper as electrode What Happens At The Electrodes During Electrolysis The products of electrolysis can be predicted for a given. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Inert electrodes should be used, otherwise they will react with the solution/products. Reactive metals are extracted from their ores using electrolysis. Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct. What Happens At The Electrodes During Electrolysis.