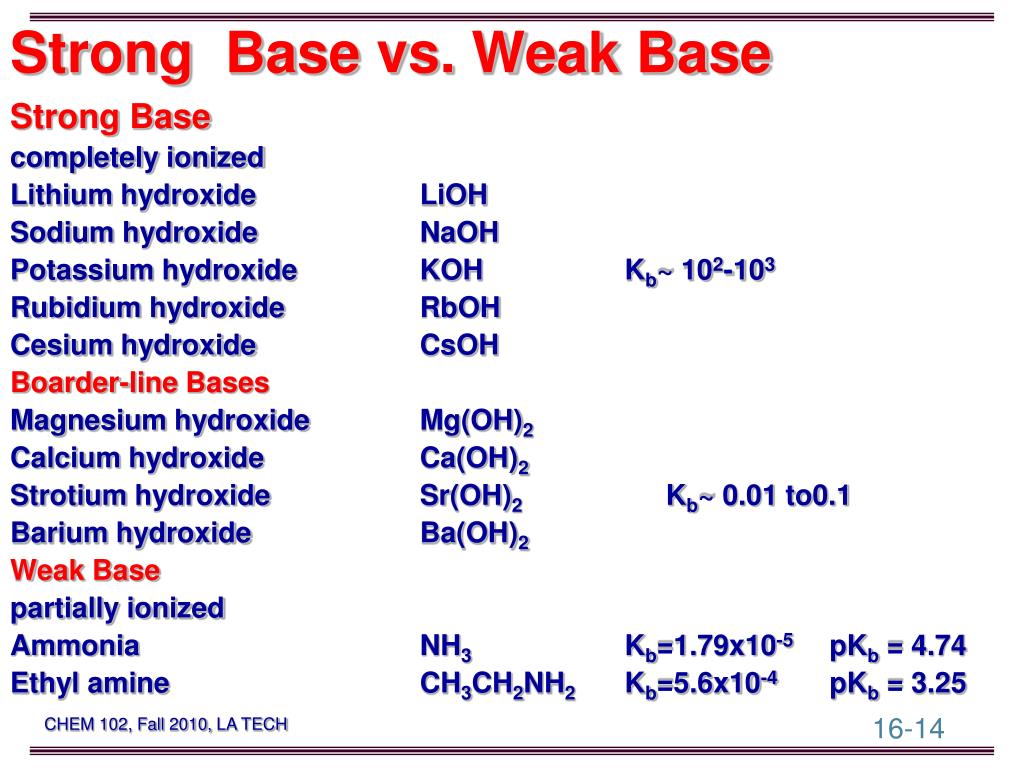
Magnesium Hydroxide Weak Or Strong Base . It is a white, crystalline solid at room temperature. i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. It is sparingly soluble in water, but its. — were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing. is magnesium hydroxide a weak base? This page explains the terms strong and weak as applied to bases. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is.
from www.slideserve.com
— magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. It is sparingly soluble in water, but its. i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. is magnesium hydroxide a weak base? since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. It is a white, crystalline solid at room temperature. — were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing. This page explains the terms strong and weak as applied to bases.
PPT Chemistry 102(01) Fall 2010 PowerPoint Presentation, free
Magnesium Hydroxide Weak Or Strong Base It is a white, crystalline solid at room temperature. This page explains the terms strong and weak as applied to bases. since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is. — were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. is magnesium hydroxide a weak base? i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. It is sparingly soluble in water, but its. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. It is a white, crystalline solid at room temperature.
From www.numerade.com
SOLVED Identifying acids and bases Identify if each of the substances Magnesium Hydroxide Weak Or Strong Base since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is. i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. This page explains the terms strong and weak as applied to bases. — magnesium hydroxide can be. Magnesium Hydroxide Weak Or Strong Base.
From www.sliderbase.com
Buffers Presentation Chemistry Magnesium Hydroxide Weak Or Strong Base It is a white, crystalline solid at room temperature. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. — were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing. since the dissociation of this small amount of dissolved magnesium hydroxide is complete,. Magnesium Hydroxide Weak Or Strong Base.
From quizdbdisfeature.z4.web.core.windows.net
Magnesium Hydroxide Formula Compound Magnesium Hydroxide Weak Or Strong Base i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. It is sparingly. Magnesium Hydroxide Weak Or Strong Base.
From courses.lumenlearning.com
Relative Strengths of Acids and Bases Chemistry for Majors Magnesium Hydroxide Weak Or Strong Base It is a white, crystalline solid at room temperature. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. It is sparingly soluble in water, but its.. Magnesium Hydroxide Weak Or Strong Base.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Magnesium Hydroxide Weak Or Strong Base — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. It is a white, crystalline solid at room temperature. It. Magnesium Hydroxide Weak Or Strong Base.
From www.studyxapp.com
identifying acids and bases identify if each of the substances when Magnesium Hydroxide Weak Or Strong Base i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. — were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing. since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide. Magnesium Hydroxide Weak Or Strong Base.
From www.researchgate.net
(a) Molecular structure diagram of magnesium hydroxide (b) Crystal Magnesium Hydroxide Weak Or Strong Base is magnesium hydroxide a weak base? Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. It is a white, crystalline solid at room temperature. — were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing. since the dissociation of this small amount of dissolved. Magnesium Hydroxide Weak Or Strong Base.
From shiken.ai
Weak Acids and Bases Magnesium Hydroxide Weak Or Strong Base i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. —. Magnesium Hydroxide Weak Or Strong Base.
From www.fishersci.co.uk
Magnesium hydroxide, 95, pure, Thermo Scientific Chemicals Fisher Magnesium Hydroxide Weak Or Strong Base since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is. — were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing. It is sparingly soluble in water, but its. It is a white, crystalline solid at room temperature. is magnesium hydroxide a weak base?. Magnesium Hydroxide Weak Or Strong Base.
From slideplayer.com
Acids & Bases. ppt download Magnesium Hydroxide Weak Or Strong Base It is a white, crystalline solid at room temperature. i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. This page explains the terms strong and weak. Magnesium Hydroxide Weak Or Strong Base.
From www.dailybody.net
Is Magnesium Hydroxide A Strong Or Weak Electrolyte? Magnesium Hydroxide Weak Or Strong Base i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial.. Magnesium Hydroxide Weak Or Strong Base.
From www.chegg.com
3. The reaction between magnesium hydroxide and Magnesium Hydroxide Weak Or Strong Base This page explains the terms strong and weak as applied to bases. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. It is sparingly soluble in water, but its. since the dissociation of this small. Magnesium Hydroxide Weak Or Strong Base.
From www.slideserve.com
PPT Chemistry 102(01) Fall 2010 PowerPoint Presentation, free Magnesium Hydroxide Weak Or Strong Base is magnesium hydroxide a weak base? This page explains the terms strong and weak as applied to bases. It is sparingly soluble in water, but its. — were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it. Magnesium Hydroxide Weak Or Strong Base.
From www.youtube.com
Is Mg(OH)2, Magnesium Hydroxide, an Acid, Base, or Neutral? YouTube Magnesium Hydroxide Weak Or Strong Base i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. It is sparingly soluble in water, but its. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. — were magnesium hydroxide a weak base, solutions of magnesium ion. Magnesium Hydroxide Weak Or Strong Base.
From topblogtenz.com
Is Ba(OH)2 strong base or weak base? Barium hydroxide Magnesium Hydroxide Weak Or Strong Base is magnesium hydroxide a weak base? Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. It is sparingly soluble in water, but its. — magnesium hydroxide can. Magnesium Hydroxide Weak Or Strong Base.
From solvedlib.com
Identifying acids and bases Identify if each of the s… SolvedLib Magnesium Hydroxide Weak Or Strong Base — were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. This page explains the terms strong and weak as applied to bases. Unlike lime, it is also much more difficult to treat magnesium. Magnesium Hydroxide Weak Or Strong Base.
From www.metabolics.com
Magnesium Hydroxide Metabolics Magnesium Hydroxide Weak Or Strong Base It is sparingly soluble in water, but its. i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. — were magnesium hydroxide a weak base, solutions. Magnesium Hydroxide Weak Or Strong Base.
From dxoygaprp.blob.core.windows.net
What Is Molecular And Ionic at Patricia Ledbetter blog Magnesium Hydroxide Weak Or Strong Base Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. is magnesium hydroxide a weak base? i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. It is a white, crystalline solid at room temperature. It is sparingly soluble. Magnesium Hydroxide Weak Or Strong Base.
From www.youtube.com
Dissociation of NaOH (A Strong Base) YouTube Magnesium Hydroxide Weak Or Strong Base It is a white, crystalline solid at room temperature. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is. This. Magnesium Hydroxide Weak Or Strong Base.
From www.garrisonminerals.com
The Industrial Side of Magnesium Hydroxide (Part 1) Magnesium Hydroxide Weak Or Strong Base It is a white, crystalline solid at room temperature. is magnesium hydroxide a weak base? since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is. i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. Unlike lime,. Magnesium Hydroxide Weak Or Strong Base.
From www.teachoo.com
Difference between Strong and Weak Base with Examples [in Table] Magnesium Hydroxide Weak Or Strong Base — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. — were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing. is magnesium hydroxide a weak base? since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide. Magnesium Hydroxide Weak Or Strong Base.
From www.youtube.com
How to Write the Formula for Magnesium hydroxide YouTube Magnesium Hydroxide Weak Or Strong Base — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. It is a white, crystalline solid at room temperature. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is. This. Magnesium Hydroxide Weak Or Strong Base.
From blog.praxilabs.com
Learn All About The Strong Acids and Bases PraxiLabs Magnesium Hydroxide Weak Or Strong Base This page explains the terms strong and weak as applied to bases. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. It is a white, crystalline solid at room temperature. — were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing. It is. Magnesium Hydroxide Weak Or Strong Base.
From www.teachoo.com
Examples of Weak Base (5 Examples with Images) Teachoo Chemistry Magnesium Hydroxide Weak Or Strong Base This page explains the terms strong and weak as applied to bases. i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. It is sparingly soluble in water, but its. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and. Magnesium Hydroxide Weak Or Strong Base.
From www.differencebetween.com
Difference Between Magnesium Oxide and Magnesium Hydroxide Compare Magnesium Hydroxide Weak Or Strong Base since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is. It is sparingly soluble in water, but its. i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. is magnesium hydroxide a weak base? This page explains. Magnesium Hydroxide Weak Or Strong Base.
From www.garrisonminerals.com
The Industrial Side of Magnesium Hydroxide (Part 1) Magnesium Hydroxide Weak Or Strong Base i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. It is sparingly soluble in water, but its. This page explains the terms strong and weak as applied to bases. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and. Magnesium Hydroxide Weak Or Strong Base.
From alevelchemistry.co.uk
Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision Magnesium Hydroxide Weak Or Strong Base It is sparingly soluble in water, but its. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. It is a white, crystalline solid at room temperature. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. This page explains the terms strong and weak as applied. Magnesium Hydroxide Weak Or Strong Base.
From www.teachoo.com
List of Strong Bases 7+ Examples Chemistry Teachoo Magnesium Hydroxide Weak Or Strong Base Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. It is a white, crystalline solid at room temperature. since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. . Magnesium Hydroxide Weak Or Strong Base.
From slideplayer.com
Chemical Bonding and Interactions ppt download Magnesium Hydroxide Weak Or Strong Base since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is. i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that is. This page explains the terms strong and weak as applied to bases. It is a white, crystalline solid. Magnesium Hydroxide Weak Or Strong Base.
From slidetodoc.com
Properties of Acids and Bases Property Acid Base Magnesium Hydroxide Weak Or Strong Base — were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. It is a white, crystalline solid at room temperature. i've seen some sources say magnesium hydroxide is a strong base, and some say that it's. Magnesium Hydroxide Weak Or Strong Base.
From dominikyouthdavidson.blogspot.com
Strong Acid and Weak Acid Magnesium Hydroxide Weak Or Strong Base — were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing. since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is. It is a white, crystalline solid at room temperature. is magnesium hydroxide a weak base? Unlike lime, it is also much more difficult. Magnesium Hydroxide Weak Or Strong Base.
From www.numerade.com
SOLVED Identifying acids and bases Identify if each of the substances Magnesium Hydroxide Weak Or Strong Base since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is. — were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. Unlike lime, it is also much. Magnesium Hydroxide Weak Or Strong Base.
From www.youtube.com
preparation and test of magnesium Hydroxide YouTube Magnesium Hydroxide Weak Or Strong Base — were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing. since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is. i've seen some sources say magnesium hydroxide is a strong base, and some say that it's a weak base, and i figured that. Magnesium Hydroxide Weak Or Strong Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2089759 Magnesium Hydroxide Weak Or Strong Base Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is. — magnesium hydroxide can be prepared through several methods, both in laboratory settings and on an industrial. This page explains the terms strong and weak as applied to bases. It is a white, crystalline solid at room temperature. — were magnesium hydroxide a. Magnesium Hydroxide Weak Or Strong Base.
From www.globeclad.com
Magnesium Hydroxide Structure Globeclad Fire Resistant Facades Magnesium Hydroxide Weak Or Strong Base This page explains the terms strong and weak as applied to bases. is magnesium hydroxide a weak base? — were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing. It is a white, crystalline solid at room temperature. Unlike lime, it is also much more difficult to treat magnesium hydroxide than. Magnesium Hydroxide Weak Or Strong Base.