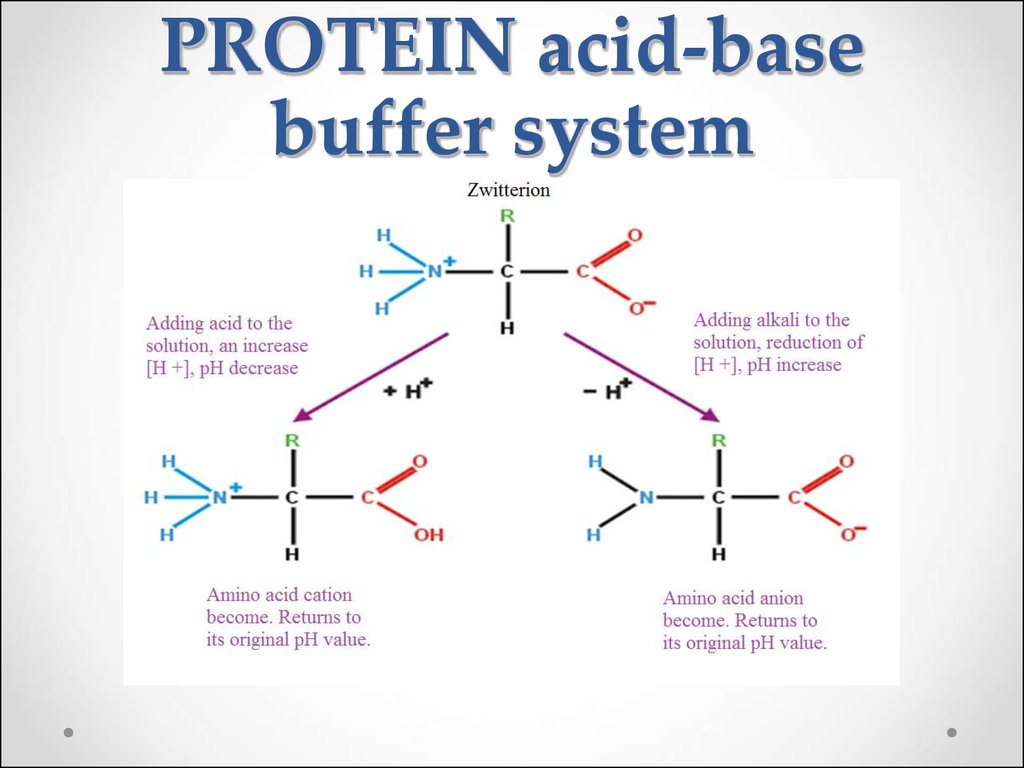
Example Of Biological Buffer System . Biological buffers are organic substances that help regulate the ph level in organisms. Biological buffers are compounds that help the body maintain a ph around 7.4. You should be aware that buffers play a critical role in almost all biochemical systems. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. And ph is no exception. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes in ph in the. Buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical for survival. Biochemical experiments routinely require a buffer. Maintenance of biological ph is important.
from en.ppt-online.org
The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes in ph in the. Maintenance of biological ph is important. Biological buffers are organic substances that help regulate the ph level in organisms. You should be aware that buffers play a critical role in almost all biochemical systems. Buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical for survival. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological buffers are compounds that help the body maintain a ph around 7.4. Biochemical experiments routinely require a buffer. And ph is no exception. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the.
Solutions. Acidbase equilibrium in biological systems online
Example Of Biological Buffer System The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. And ph is no exception. Biological buffers are organic substances that help regulate the ph level in organisms. Maintenance of biological ph is important. Buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical for survival. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. Biochemical experiments routinely require a buffer. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes in ph in the. Biological buffers are compounds that help the body maintain a ph around 7.4. You should be aware that buffers play a critical role in almost all biochemical systems. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.
From loeukppln.blob.core.windows.net
Buffers Ap Biology at Venessa Baird blog Example Of Biological Buffer System Buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical for survival. Biological buffers are organic substances that help regulate the ph level in organisms. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. Biochemical experiments routinely require a buffer. Biological buffers are. Example Of Biological Buffer System.
From www.slideserve.com
PPT Chapter 2 Water Lehninger Principles of Biochemistry, Fourth Example Of Biological Buffer System You should be aware that buffers play a critical role in almost all biochemical systems. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. And ph is no exception. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological buffers are. Example Of Biological Buffer System.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Example Of Biological Buffer System Biological buffers are organic substances that help regulate the ph level in organisms. Maintenance of biological ph is important. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. You should be aware that buffers play a critical role in almost all biochemical systems. Buffers play an immense role in biological systems where. Example Of Biological Buffer System.
From www.slideshare.net
Buffers in biological systems PPT Example Of Biological Buffer System Maintenance of biological ph is important. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. And ph is no exception. Biochemical experiments routinely require a buffer. Biological buffers are organic substances that help regulate the ph level in organisms. The purpose of a buffer in a biological system is to maintain intracellular. Example Of Biological Buffer System.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 Example Of Biological Buffer System You should be aware that buffers play a critical role in almost all biochemical systems. Buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical for survival. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Example Of Biological Buffer System.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation Example Of Biological Buffer System The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. And ph is no exception. Buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical for survival. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow. Example Of Biological Buffer System.
From joifezsdx.blob.core.windows.net
Biological Buffers Ppt at Erin Dodds blog Example Of Biological Buffer System The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. You should be aware that buffers play a critical role in almost all biochemical systems. And ph is no exception. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. Biological buffers are. Example Of Biological Buffer System.
From dxouambuf.blob.core.windows.net
How To Use A Buffer at Albert Hill blog Example Of Biological Buffer System Biological buffers are organic substances that help regulate the ph level in organisms. Maintenance of biological ph is important. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes in ph in the. Biochemical experiments routinely require a buffer. And ph is no exception. The most. Example Of Biological Buffer System.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online Example Of Biological Buffer System And ph is no exception. Biological buffers are organic substances that help regulate the ph level in organisms. Maintenance of biological ph is important. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. Biological buffers are compounds that help the body maintain a ph around 7.4. Buffers play an. Example Of Biological Buffer System.
From www.leinco.com
Biological Buffers And pH Range Leinco Technologies Example Of Biological Buffer System Maintenance of biological ph is important. Biological buffers are organic substances that help regulate the ph level in organisms. Biochemical experiments routinely require a buffer. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes in ph in the. Biological buffers are compounds that help the. Example Of Biological Buffer System.
From www.studypool.com
SOLUTION Biological buffer systems Studypool Example Of Biological Buffer System Biochemical experiments routinely require a buffer. Buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical for survival. Maintenance of biological ph is important. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The purpose of a buffer in a biological system is. Example Of Biological Buffer System.
From www.slideserve.com
PPT CHAPTER 2 Water and Aqueous Solutions PowerPoint Presentation Example Of Biological Buffer System Biochemical experiments routinely require a buffer. And ph is no exception. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known. Example Of Biological Buffer System.
From study.com
Buffer System in Chemistry Definition & Overview Video & Lesson Example Of Biological Buffer System You should be aware that buffers play a critical role in almost all biochemical systems. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes in ph in the.. Example Of Biological Buffer System.
From www.youtube.com
Role of buffers in biological system YouTube Example Of Biological Buffer System The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes in ph in the. Biological buffers are organic substances that help regulate the ph level in organisms. You should. Example Of Biological Buffer System.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Example Of Biological Buffer System The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. You should be aware that buffers play a critical role in almost all biochemical systems. And ph is no exception. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. Maintenance of biological ph is important.. Example Of Biological Buffer System.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and Example Of Biological Buffer System Biological buffers are organic substances that help regulate the ph level in organisms. And ph is no exception. Buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical for survival. Maintenance of biological ph is important. The purpose of a buffer in a biological system is to maintain intracellular and extracellular. Example Of Biological Buffer System.
From www.reagent.co.uk
How Do Biological Buffers Work? The Science Blog Example Of Biological Buffer System The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. And ph is no exception. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. Buffers. Example Of Biological Buffer System.
From www.slideshare.net
02 hydrolysis. buffers__colloids Example Of Biological Buffer System The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. Buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical for survival. The purpose. Example Of Biological Buffer System.
From dxobuticl.blob.core.windows.net
Commonly Used Buffers In The Laboratory at Savannah Osgood blog Example Of Biological Buffer System The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. Biological buffers are organic substances that help regulate the ph level in organisms. They act by neutralizing excess hydrogen ions, thereby maintaining the. Example Of Biological Buffer System.
From www.youtube.com
Acid, Base & Buffer in Biological systems YouTube Example Of Biological Buffer System The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical for survival. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and. Example Of Biological Buffer System.
From www.youtube.com
Buffer Capacity 03 Buffers in pharmaceutical and biological systems Example Of Biological Buffer System The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes in ph in the. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological buffers are organic substances that help regulate the ph level in organisms. And ph. Example Of Biological Buffer System.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching Example Of Biological Buffer System And ph is no exception. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. Biological buffers are compounds that help the body maintain a ph around 7.4. Biological buffers are organic substances. Example Of Biological Buffer System.
From www.slideshare.net
Buffers in biological systems PPT Example Of Biological Buffer System And ph is no exception. You should be aware that buffers play a critical role in almost all biochemical systems. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. Biological buffers are organic substances that help regulate the ph level in organisms. Biochemical experiments routinely require a buffer. The buffer systems functioning. Example Of Biological Buffer System.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free Example Of Biological Buffer System The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. Biochemical experiments routinely require a buffer. Biological buffers are organic substances that help regulate the ph level in organisms. Maintenance of biological ph is important. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and. Example Of Biological Buffer System.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Example Of Biological Buffer System Maintenance of biological ph is important. You should be aware that buffers play a critical role in almost all biochemical systems. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. Buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical for survival. Biochemical. Example Of Biological Buffer System.
From www.slideserve.com
PPT THEME Acidbase equilibrium in biological systems. Buffer Example Of Biological Buffer System The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. Biological buffers are organic substances that help regulate the ph level in organisms. Biochemical experiments routinely require a buffer. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. You should be aware. Example Of Biological Buffer System.
From www.scribd.com
Biological Buffer System Buffer Solution Ph Example Of Biological Buffer System The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological buffers are organic substances that help regulate the ph level in organisms. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. You should be aware that buffers play a critical role. Example Of Biological Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Example Of Biological Buffer System The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes in ph in the. Biological buffers are organic substances that help regulate the ph level in organisms. Biochemical experiments routinely require a buffer. Buffers play an immense role in biological systems where maintaining constant internal conditions,. Example Of Biological Buffer System.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Example Of Biological Buffer System You should be aware that buffers play a critical role in almost all biochemical systems. And ph is no exception. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. Buffers play an immense role in biological systems where maintaining constant internal conditions, otherwise known as homeostasis, is critical for. Example Of Biological Buffer System.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Example Of Biological Buffer System And ph is no exception. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. Biochemical experiments routinely require a buffer. You should be aware that buffers play a critical role in almost all biochemical systems. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow. Example Of Biological Buffer System.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Example Of Biological Buffer System Biochemical experiments routinely require a buffer. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. Maintenance of biological ph is important. They act by neutralizing excess hydrogen ions, thereby maintaining the ph. Example Of Biological Buffer System.
From www.slideshare.net
Buffer system Example Of Biological Buffer System The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes in ph in the. Maintenance of biological ph is important. Biological buffers are organic substances that help regulate the ph level in organisms. Buffers play an immense role in biological systems where maintaining constant internal conditions,. Example Of Biological Buffer System.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Example Of Biological Buffer System Biological buffers are compounds that help the body maintain a ph around 7.4. And ph is no exception. Maintenance of biological ph is important. Biochemical experiments routinely require a buffer. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. You should be aware that buffers play a critical role in almost all. Example Of Biological Buffer System.
From www.slideshare.net
Buffers in biological systems PPT Example Of Biological Buffer System The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological buffers are compounds that help the body maintain a ph around 7.4. They act by neutralizing excess hydrogen ions, thereby maintaining the. Example Of Biological Buffer System.
From www.youtube.com
buffer isotonic solution buffer capacity buffers in pharmaceutical Example Of Biological Buffer System The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes in ph in the. Biological buffers are compounds that help the body maintain a ph around. Example Of Biological Buffer System.