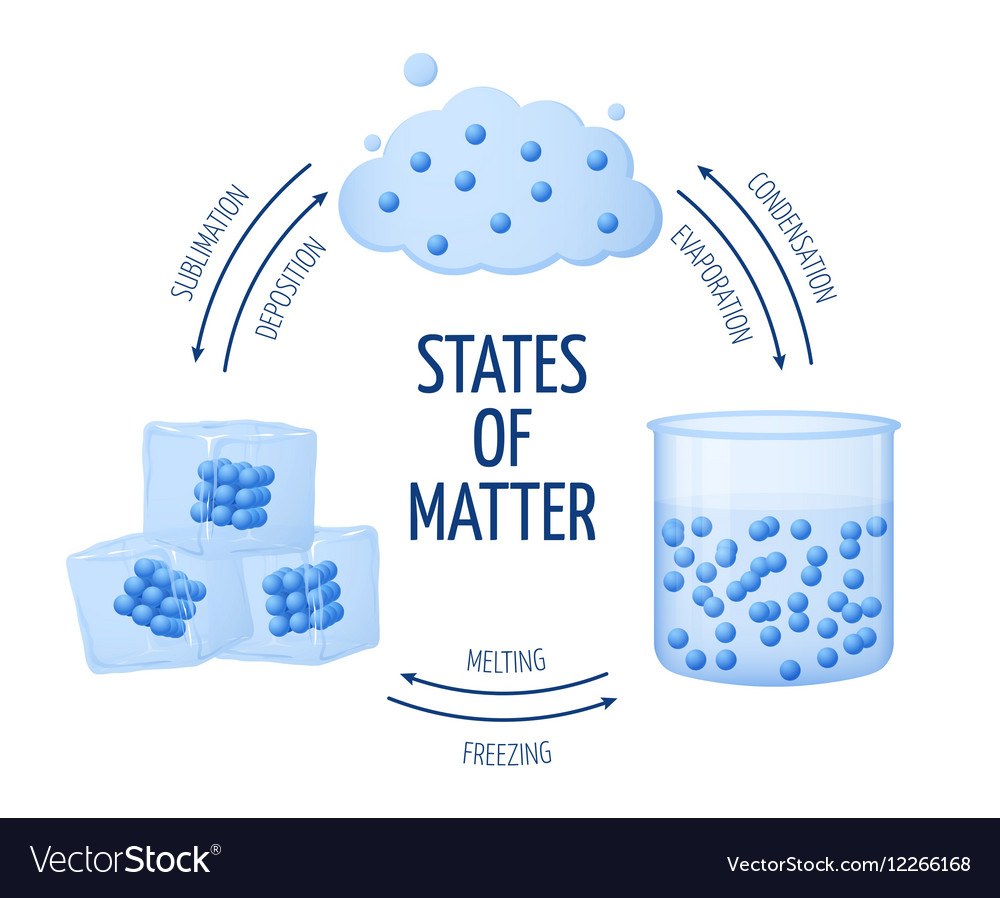
Explain Density Of Solid Liquid Gas Forms Of Water . The solid close solid one of the three states. Explain the relationship between pressure. There are 3 different forms of water, or h 2 o: Liquid water has a density of 1.00 g/cm³. Learn how density is different for solids, liquids, gasses and different materials. Compare and contrast the densities of various substances. Table 14.2 shows the density of water in various phases and temperature. Air (in its gaseous form) has a density of 0.0012 g/cm³. Solid steel has a density of 7.82 g/cm³. The density of water is 1. The density of solids and liquids normally increase with decreasing temperature. Define pressure and its related si units. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Solid (ice), liquid (water), and gas (steam). Just like a solid, the density of a liquid equals the mass of the liquid divided by its volume;
from www.vectorstock.com
Water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: Liquid water has a density of 1.00 g/cm³. Just like a solid, the density of a liquid equals the mass of the liquid divided by its volume; Because water seems so ubiquitous, many people are. And why they adopt the shape (but not. The density of solids and liquids normally increase with decreasing temperature. The water molecules are pushed farther apart. The thermal expansion of liquids; Learn how density is different for solids, liquids, gasses and different materials. Air (in its gaseous form) has a density of 0.0012 g/cm³.
Different states of matter solid liquid gas Vector Image
Explain Density Of Solid Liquid Gas Forms Of Water The density of solids and liquids normally increase with decreasing temperature. Compare and contrast the densities of various substances. There are 3 different forms of water, or h 2 o: Define pressure and its related si units. Learn how density is different for solids, liquids, gasses and different materials. And why they adopt the shape (but not. Table 14.2 shows the density of water in various phases and temperature. Solid steel has a density of 7.82 g/cm³. The solid close solid one of the three states. Explain the relationship between pressure. Solid (ice), liquid (water), and gas (steam). Just like a solid, the density of a liquid equals the mass of the liquid divided by its volume; Water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: Because water seems so ubiquitous, many people are. Air (in its gaseous form) has a density of 0.0012 g/cm³. The water molecules are pushed farther apart.
From www.alamy.com
Density and states of matter. vector diagram compares the particles in Explain Density Of Solid Liquid Gas Forms Of Water The density of water is 1. Just like a solid, the density of a liquid equals the mass of the liquid divided by its volume; Compare and contrast the densities of various substances. The density of solids and liquids normally increase with decreasing temperature. Table 14.2 shows the density of water in various phases and temperature. The water molecules are. Explain Density Of Solid Liquid Gas Forms Of Water.
From vdocuments.mx
Remember Water can be a liquid or a solid or a gas. Solids, liquids and Explain Density Of Solid Liquid Gas Forms Of Water Compare and contrast the densities of various substances. Learn how density is different for solids, liquids, gasses and different materials. The thermal expansion of liquids; Table 14.2 shows the density of water in various phases and temperature. Solid (ice), liquid (water), and gas (steam). Water’s lower density in its solid form is due to the way hydrogen bonds are oriented. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.snexplores.org
Explainer What are the different states of matter? Explain Density Of Solid Liquid Gas Forms Of Water Air (in its gaseous form) has a density of 0.0012 g/cm³. Explain the relationship between pressure. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Revise the equation for density and try some example questions. Define pressure and its related si units. Water’s lower density in its solid form is due to the way. Explain Density Of Solid Liquid Gas Forms Of Water.
From allaboutchemistry123.blogspot.com
What are solid, liquid, and gases? All About Chemistry Explain Density Of Solid Liquid Gas Forms Of Water Explain the relationship between pressure. Learn how density is different for solids, liquids, gasses and different materials. And why they adopt the shape (but not. Solid steel has a density of 7.82 g/cm³. The density of solids and liquids normally increase with decreasing temperature. There are 3 different forms of water, or h 2 o: Because water seems so ubiquitous,. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.alamy.com
matter in different states for example water. solid, liquid, gas and Explain Density Of Solid Liquid Gas Forms Of Water Water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: The thermal expansion of liquids; Air (in its gaseous form) has a density of 0.0012 g/cm³. Liquid water has a density of 1.00 g/cm³. Solid steel has a density of 7.82 g/cm³. The solid close solid one of the three states.. Explain Density Of Solid Liquid Gas Forms Of Water.
From sciencenotes.org
States of Matter Explain Density Of Solid Liquid Gas Forms Of Water Table 14.2 shows the density of water in various phases and temperature. The density of solids and liquids normally increase with decreasing temperature. Compare and contrast the densities of various substances. The water molecules are pushed farther apart. Water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: Solid steel has. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Explain Density Of Solid Liquid Gas Forms Of Water The solid close solid one of the three states. Liquid water has a density of 1.00 g/cm³. Solid steel has a density of 7.82 g/cm³. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Define pressure and its related si units. Table 14.2 shows the density of water in various phases and temperature. The. Explain Density Of Solid Liquid Gas Forms Of Water.
From sayngon.com
Are Most Liquids Denser Than Solids? Exploring Density Differences Explain Density Of Solid Liquid Gas Forms Of Water The water molecules are pushed farther apart. And why they adopt the shape (but not. Liquid water has a density of 1.00 g/cm³. The solid close solid one of the three states. Explain the relationship between pressure. Revise the equation for density and try some example questions. Compare and contrast the densities of various substances. Water’s lower density in its. Explain Density Of Solid Liquid Gas Forms Of Water.
From dxojwesjc.blob.core.windows.net
Lesson Plan Properties Of Solids Liquids And Gases at Albert Mitchell blog Explain Density Of Solid Liquid Gas Forms Of Water Liquid water has a density of 1.00 g/cm³. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; There are 3 different forms of water, or h 2 o: The solid close solid one of the three states. The density of solids and liquids normally increase with decreasing temperature. Because water seems so ubiquitous, many. Explain Density Of Solid Liquid Gas Forms Of Water.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Explain Density Of Solid Liquid Gas Forms Of Water Compare and contrast the densities of various substances. Air (in its gaseous form) has a density of 0.0012 g/cm³. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Learn how density is different for solids, liquids, gasses and different materials. The solid close solid one of the three states. Because water seems so ubiquitous,. Explain Density Of Solid Liquid Gas Forms Of Water.
From learningnadeauobtuser.z21.web.core.windows.net
How To Determine Density Of A Liquid Explain Density Of Solid Liquid Gas Forms Of Water The thermal expansion of liquids; Water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: The density of solids and liquids normally increase with decreasing temperature. Learn how density is different for solids, liquids, gasses and different materials. The density of water is 1. Solid (ice), liquid (water), and gas (steam).. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock Explain Density Of Solid Liquid Gas Forms Of Water Define pressure and its related si units. Liquid water has a density of 1.00 g/cm³. Solid steel has a density of 7.82 g/cm³. The solid close solid one of the three states. Compare and contrast the densities of various substances. Water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: Revise. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.radixtree.com
Physics Matter Online Education System Explain Density Of Solid Liquid Gas Forms Of Water The water molecules are pushed farther apart. Just like a solid, the density of a liquid equals the mass of the liquid divided by its volume; Compare and contrast the densities of various substances. Air (in its gaseous form) has a density of 0.0012 g/cm³. And why they adopt the shape (but not. Table 14.2 shows the density of water. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.britannica.com
phase Definition & Facts Britannica Explain Density Of Solid Liquid Gas Forms Of Water Revise the equation for density and try some example questions. Learn how density is different for solids, liquids, gasses and different materials. Solid steel has a density of 7.82 g/cm³. Liquid water has a density of 1.00 g/cm³. The density of water is 1. Table 14.2 shows the density of water in various phases and temperature. Just like a solid,. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.dreamstime.com
States of Matter. Vector Circles Infographic Illustration Stock Vector Explain Density Of Solid Liquid Gas Forms Of Water Explain the relationship between pressure. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Revise the equation for density and try some example questions. The density of water is 1. And why they adopt the shape (but not. Because water seems so ubiquitous, many people are. The density of solids and liquids normally increase. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.tutorix.com
Give three characteristics of solid liquid and gas Tutorix Explain Density Of Solid Liquid Gas Forms Of Water Water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: The water molecules are pushed farther apart. The thermal expansion of liquids; And why they adopt the shape (but not. Learn how density is different for solids, liquids, gasses and different materials. The solid close solid one of the three states.. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.alamy.com
Density of matter with gas, liquid and solid water states outline Explain Density Of Solid Liquid Gas Forms Of Water Solid (ice), liquid (water), and gas (steam). The solid close solid one of the three states. Table 14.2 shows the density of water in various phases and temperature. There are 3 different forms of water, or h 2 o: Explain the relationship between pressure. Just like a solid, the density of a liquid equals the mass of the liquid divided. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.expii.com
Unique Densities (Water) — Properties & Examples Expii Explain Density Of Solid Liquid Gas Forms Of Water Because water seems so ubiquitous, many people are. The thermal expansion of liquids; The water molecules are pushed farther apart. Learn how density is different for solids, liquids, gasses and different materials. Table 14.2 shows the density of water in various phases and temperature. Just like a solid, the density of a liquid equals the mass of the liquid divided. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.alamy.com
fundamental states of matter. Density and molecular structure of Solid Explain Density Of Solid Liquid Gas Forms Of Water The density of water is 1. Compare and contrast the densities of various substances. Liquid water has a density of 1.00 g/cm³. Because water seems so ubiquitous, many people are. Define pressure and its related si units. The density of solids and liquids normally increase with decreasing temperature. Table 14.2 shows the density of water in various phases and temperature.. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Explain Density Of Solid Liquid Gas Forms Of Water There are 3 different forms of water, or h 2 o: Revise the equation for density and try some example questions. Explain the relationship between pressure. Solid steel has a density of 7.82 g/cm³. Liquid water has a density of 1.00 g/cm³. Table 14.2 shows the density of water in various phases and temperature. This model explains the higher density,. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.youtube.com
States of matter 🚗💧☁️ Solid, Liquid & Gas Learn with examples YouTube Explain Density Of Solid Liquid Gas Forms Of Water Define pressure and its related si units. The thermal expansion of liquids; Learn how density is different for solids, liquids, gasses and different materials. The water molecules are pushed farther apart. Table 14.2 shows the density of water in various phases and temperature. Water’s lower density in its solid form is due to the way hydrogen bonds are oriented as. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.vectorstock.com
Different states of matter solid liquid gas Vector Image Explain Density Of Solid Liquid Gas Forms Of Water Water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: The solid close solid one of the three states. The water molecules are pushed farther apart. Define pressure and its related si units. There are 3 different forms of water, or h 2 o: Compare and contrast the densities of various. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.youtube.com
States of Matter Solid, Liquid, Gases. Interesting Animated Lesson Explain Density Of Solid Liquid Gas Forms Of Water Compare and contrast the densities of various substances. The solid close solid one of the three states. Just like a solid, the density of a liquid equals the mass of the liquid divided by its volume; Because water seems so ubiquitous, many people are. The water molecules are pushed farther apart. Explain the relationship between pressure. There are 3 different. Explain Density Of Solid Liquid Gas Forms Of Water.
From mavink.com
Density Of Solids Liquids And Gases Explain Density Of Solid Liquid Gas Forms Of Water The density of solids and liquids normally increase with decreasing temperature. Just like a solid, the density of a liquid equals the mass of the liquid divided by its volume; Water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: Learn how density is different for solids, liquids, gasses and different. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Explain Density Of Solid Liquid Gas Forms Of Water The solid close solid one of the three states. Liquid water has a density of 1.00 g/cm³. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Revise the equation for density and try some example questions. Just like a solid, the density of a liquid equals the mass of the liquid divided by its. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.teachoo.com
Difference between Solid, Liquid, Gas in Table Form Teachoo Explain Density Of Solid Liquid Gas Forms Of Water Explain the relationship between pressure. Air (in its gaseous form) has a density of 0.0012 g/cm³. The solid close solid one of the three states. Water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: And why they adopt the shape (but not. Table 14.2 shows the density of water in. Explain Density Of Solid Liquid Gas Forms Of Water.
From joisrkcqc.blob.core.windows.net
Which Properties Do All Solids Liquids And Gases Have at Estelle Lewis blog Explain Density Of Solid Liquid Gas Forms Of Water Explain the relationship between pressure. There are 3 different forms of water, or h 2 o: Just like a solid, the density of a liquid equals the mass of the liquid divided by its volume; Learn how density is different for solids, liquids, gasses and different materials. And why they adopt the shape (but not. Solid steel has a density. Explain Density Of Solid Liquid Gas Forms Of Water.
From exycfidyi.blob.core.windows.net
Density Of Solid Liquid And Gas Class 9 at Megan Shearer blog Explain Density Of Solid Liquid Gas Forms Of Water Revise the equation for density and try some example questions. Solid (ice), liquid (water), and gas (steam). This model explains the higher density, greater order, and lower compressibility of liquids versus gases; There are 3 different forms of water, or h 2 o: The density of water is 1. Just like a solid, the density of a liquid equals the. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.slideserve.com
PPT Earth ’ s Water Cycle for Elementary School Students PowerPoint Explain Density Of Solid Liquid Gas Forms Of Water Solid steel has a density of 7.82 g/cm³. Revise the equation for density and try some example questions. Define pressure and its related si units. The density of water is 1. The thermal expansion of liquids; There are 3 different forms of water, or h 2 o: Because water seems so ubiquitous, many people are. This model explains the higher. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.youtube.com
States of Matter Solid Liquid Gas States of Matter drawing Different Explain Density Of Solid Liquid Gas Forms Of Water This model explains the higher density, greater order, and lower compressibility of liquids versus gases; The water molecules are pushed farther apart. Learn how density is different for solids, liquids, gasses and different materials. Compare and contrast the densities of various substances. Air (in its gaseous form) has a density of 0.0012 g/cm³. The density of water is 1. There. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.dreamstime.com
Density of Matter with Gas, Liquid and Solid Water States Outline Explain Density Of Solid Liquid Gas Forms Of Water Define pressure and its related si units. The density of solids and liquids normally increase with decreasing temperature. Water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: Compare and contrast the densities of various substances. The thermal expansion of liquids; The solid close solid one of the three states. Learn. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.grc.nasa.gov
Phases of Matter Explain Density Of Solid Liquid Gas Forms Of Water Solid steel has a density of 7.82 g/cm³. Because water seems so ubiquitous, many people are. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Air (in its gaseous form) has a density of 0.0012 g/cm³. The water molecules are pushed farther apart. Table 14.2 shows the density of water in various phases and. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.dreamstime.com
Density of Matter with Gas, Liquid and Solid Particle States Outline Explain Density Of Solid Liquid Gas Forms Of Water Explain the relationship between pressure. Define pressure and its related si units. Solid (ice), liquid (water), and gas (steam). The solid close solid one of the three states. The density of water is 1. The density of solids and liquids normally increase with decreasing temperature. There are 3 different forms of water, or h 2 o: And why they adopt. Explain Density Of Solid Liquid Gas Forms Of Water.
From owlcation.com
All About Matter An Introduction to the Basics Owlcation Explain Density Of Solid Liquid Gas Forms Of Water The density of water is 1. Table 14.2 shows the density of water in various phases and temperature. Water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: Define pressure and its related si units. The water molecules are pushed farther apart. Explain the relationship between pressure. The density of solids. Explain Density Of Solid Liquid Gas Forms Of Water.
From www.dreamstime.com
Density and States of Matter Stock Vector Illustration of science Explain Density Of Solid Liquid Gas Forms Of Water Explain the relationship between pressure. There are 3 different forms of water, or h 2 o: The density of solids and liquids normally increase with decreasing temperature. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Table 14.2 shows the density of water in various phases and temperature. Learn how density is different for. Explain Density Of Solid Liquid Gas Forms Of Water.