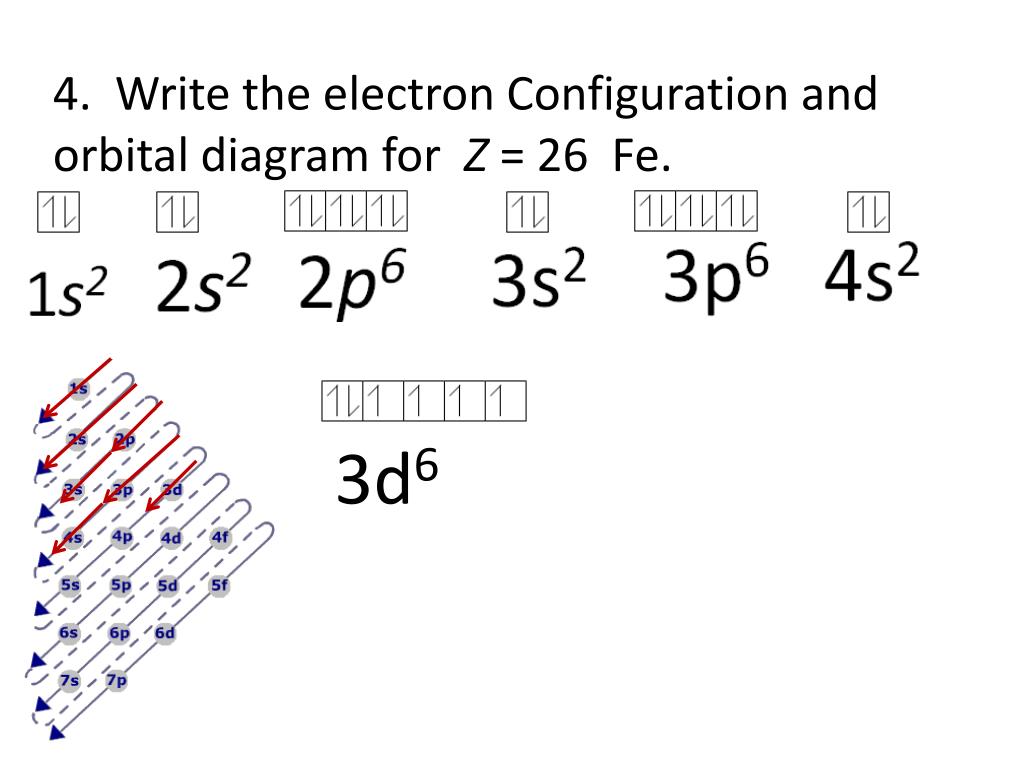
Fe Outermost Electrons . Iron can also give up either of the paired electrons from a 3d. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. In layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. As previously stated, iron has two valence states: Valence electrons are those in the outermost principal energy level. For example, sodium has one valence electron, and chlorine has seven valence electrons: How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. When we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that. Iron has 26 electrons so its normal electron configuration would be: In the periodic table, elements with analogous valence electron configurations usually occur within the same group. As a result, when it gives up the two 4s electrons, it gains a valency of +2. Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. But for most of the transition and inner.
from www.slideserve.com
Iron has 26 electrons so its normal electron configuration would be: For example, sodium has one valence electron, and chlorine has seven valence electrons: As previously stated, iron has two valence states: Iron can also give up either of the paired electrons from a 3d. When we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that. In layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. But for most of the transition and inner.
PPT More on Electron Configurations PowerPoint Presentation, free
Fe Outermost Electrons Iron can also give up either of the paired electrons from a 3d. For example, sodium has one valence electron, and chlorine has seven valence electrons: Iron has 26 electrons so its normal electron configuration would be: As previously stated, iron has two valence states: As a result, when it gives up the two 4s electrons, it gains a valency of +2. Valence electrons are those in the outermost principal energy level. Iron can also give up either of the paired electrons from a 3d. Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. But for most of the transition and inner. When we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. In the periodic table, elements with analogous valence electron configurations usually occur within the same group. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. In layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely.
From www.slideserve.com
PPT More on Electron Configurations PowerPoint Presentation, free Fe Outermost Electrons But for most of the transition and inner. In layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. As previously stated, iron has two valence states: Iron can also give up either of the paired electrons from a 3d. Electrons in the outermost orbitals, called valence electrons,. Fe Outermost Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Fe Outermost Electrons In layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. Valence electrons are those in the outermost principal energy level. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. But for most of the transition and inner. As a result, when. Fe Outermost Electrons.
From loeqsufar.blob.core.windows.net
Fe Element Electrons at Jack Nguyen blog Fe Outermost Electrons Iron can also give up either of the paired electrons from a 3d. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. In. Fe Outermost Electrons.
From saylordotorg.github.io
Building Up the Periodic Table Fe Outermost Electrons For example, sodium has one valence electron, and chlorine has seven valence electrons: Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. Iron has 26 electrons so its normal electron configuration would be: As a result,. Fe Outermost Electrons.
From www.scienceabc.com
What Are Valence Electrons And How To Find Them? Where Are They Located? Fe Outermost Electrons As a result, when it gives up the two 4s electrons, it gains a valency of +2. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. Valence electrons are those in the outermost principal energy level. In the periodic table, elements. Fe Outermost Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Fe Outermost Electrons As previously stated, iron has two valence states: For example, sodium has one valence electron, and chlorine has seven valence electrons: When we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we. Fe Outermost Electrons.
From www.youtube.com
Valence Electrons for Fe (Iron) YouTube Fe Outermost Electrons Iron has 26 electrons so its normal electron configuration would be: But for most of the transition and inner. When we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that. Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. As previously. Fe Outermost Electrons.
From www.slideserve.com
PPT Chemistry for Bio 9 PowerPoint Presentation, free download ID Fe Outermost Electrons But for most of the transition and inner. Iron has 26 electrons so its normal electron configuration would be: Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. In the periodic table, elements with analogous valence electron configurations usually occur within the same group. In layman’s terms, the number of electrons. Fe Outermost Electrons.
From www.numerade.com
SOLVED18. Write out the electron configuration for Fe" , Fe'? and Fe Fe Outermost Electrons Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Iron can also give up either of the paired electrons from a 3d. As previously stated, iron has two valence states: Valence electrons are those in the outermost principal energy level. Electrons in the outermost orbitals, called valence electrons, are responsible for most of the. Fe Outermost Electrons.
From slidetodoc.com
Calculating Particles for an ion Representations from the Fe Outermost Electrons Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. As a result, when it gives up the two 4s electrons, it gains a valency of +2.. Fe Outermost Electrons.
From www.schoolmykids.com
Iron (Fe) Element Information, Facts, Properties, Uses Periodic Fe Outermost Electrons When we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that. For example, sodium has one valence electron, and chlorine has seven valence electrons: Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. In the periodic table, elements with analogous valence. Fe Outermost Electrons.
From praxilabs.com
Your Step by Step Guide to Find Valence Electrons praxilabs Fe Outermost Electrons When we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. In the periodic table, elements with analogous valence electron configurations usually. Fe Outermost Electrons.
From ar.inspiredpencil.com
Iron Orbital Notation Fe Outermost Electrons As previously stated, iron has two valence states: In the periodic table, elements with analogous valence electron configurations usually occur within the same group. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. When we make a 3+ ion for iron,. Fe Outermost Electrons.
From surfguppy.com
Valence Electrons Definition, Obits and Energy Level Fe Outermost Electrons In layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. But for most of the transition and inner. When we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that. In the periodic table, elements with analogous valence. Fe Outermost Electrons.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind Fe Outermost Electrons Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. But for most of the transition and inner. For example, sodium has one valence electron, and chlorine has seven valence electrons: Valence electrons are those in the outermost principal energy level. When we make a 3+ ion for iron, we need to take the electrons. Fe Outermost Electrons.
From www.wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Fe Outermost Electrons In layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Iron can also give up either of the paired electrons from a 3d. For example, sodium has one valence electron, and chlorine. Fe Outermost Electrons.
From www.slideserve.com
PPT Entry Task Oct 22 nd Monday PowerPoint Presentation, free Fe Outermost Electrons When we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that. As previously stated, iron has two valence states: As a result, when it gives up the two 4s electrons, it gains a valency of +2. But for most of the transition and inner. How to write the electron configuration. Fe Outermost Electrons.
From discover.lanl.gov
What are valence orbitals? Discover Los Alamos National Laboratory Fe Outermost Electrons As a result, when it gives up the two 4s electrons, it gains a valency of +2. But for most of the transition and inner. Iron can also give up either of the paired electrons from a 3d. As previously stated, iron has two valence states: Electrons in the outermost orbitals, called valence electrons, are responsible for most of the. Fe Outermost Electrons.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Fe Outermost Electrons Iron can also give up either of the paired electrons from a 3d. For example, sodium has one valence electron, and chlorine has seven valence electrons: In layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. Fe 1s 2 2s 2 2p 6 3s 2 3p 6. Fe Outermost Electrons.
From www.slideserve.com
PPT Atom video PowerPoint Presentation ID386307 Fe Outermost Electrons Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Iron has 26 electrons so its normal electron configuration would be: Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. As previously stated, iron has two valence states: Iron can also give up either of the. Fe Outermost Electrons.
From chem.libretexts.org
6.4 Electronic Structure of Atoms (Electron Configurations Fe Outermost Electrons As a result, when it gives up the two 4s electrons, it gains a valency of +2. But for most of the transition and inner. As previously stated, iron has two valence states: Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. For example, sodium has one valence electron, and chlorine. Fe Outermost Electrons.
From elitetutors.in
Chemistry Chapter 4. Structure of Atom Fe Outermost Electrons Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. When we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. For example, sodium has one valence. Fe Outermost Electrons.
From www.shutterstock.com
46 Fe Electron Configurations Images, Stock Photos & Vectors Shutterstock Fe Outermost Electrons For example, sodium has one valence electron, and chlorine has seven valence electrons: Valence electrons are those in the outermost principal energy level. As previously stated, iron has two valence states: Iron has 26 electrons so its normal electron configuration would be: But for most of the transition and inner. Fe 1s 2 2s 2 2p 6 3s 2 3p. Fe Outermost Electrons.
From sciencenotes.org
Periodic Table Outermost Electron Orbitals Fe Outermost Electrons In layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. When we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that. For example, sodium has one valence electron, and chlorine has seven valence electrons: As a result,. Fe Outermost Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Fe Outermost Electrons Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. In the periodic table, elements with analogous valence electron configurations usually occur within the same group. For example, sodium has one valence electron, and chlorine has seven valence electrons: When we make a 3+ ion for iron, we need to take the. Fe Outermost Electrons.
From sciencenotes.org
List of Electron Configurations of Elements Fe Outermost Electrons In the periodic table, elements with analogous valence electron configurations usually occur within the same group. As a result, when it gives up the two 4s electrons, it gains a valency of +2. Iron has 26 electrons so its normal electron configuration would be: How to write the electron configuration for iron (fe) in order to write the iron electron. Fe Outermost Electrons.
From chem.libretexts.org
Electronic Configurations Intro Chemistry LibreTexts Fe Outermost Electrons When we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that. As a result, when it gives up the two 4s electrons, it gains a valency of +2. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. In layman’s terms, the number of electrons. Fe Outermost Electrons.
From slideplayer.com
Chemical Bonding. ppt download Fe Outermost Electrons Iron has 26 electrons so its normal electron configuration would be: Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. Iron can also give up either of the paired electrons from a 3d. As previously stated, iron has two valence states: For example, sodium has one valence electron, and chlorine has. Fe Outermost Electrons.
From www.allaboutcircuits.com
Valence and Crystal Structure Solidstate Device Theory Electronics Fe Outermost Electrons Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. As a result, when it gives up the two 4s electrons, it gains a valency of +2. But for most of the transition and inner. In the periodic table, elements with analogous valence electron configurations usually occur within the same group. For. Fe Outermost Electrons.
From periodictable.me
Valence+Electrons+The+electrons+on+the+outermost+shell+of+an+atom Fe Outermost Electrons As previously stated, iron has two valence states: For example, sodium has one valence electron, and chlorine has seven valence electrons: Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. Iron has 26 electrons so its normal electron configuration would be: In the periodic table, elements with analogous valence electron configurations. Fe Outermost Electrons.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica Fe Outermost Electrons How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. For example, sodium has one valence electron, and chlorine has seven valence electrons: Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. Iron. Fe Outermost Electrons.
From slideplayer.com
Bonds, Chemical Bonds Chapter ppt download Fe Outermost Electrons Iron can also give up either of the paired electrons from a 3d. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. As a result, when it gives up the two 4s electrons, it gains a valency of +2. In layman’s. Fe Outermost Electrons.
From www.nagwa.com
Question Video Identifying the Number of Electrons in the Outermost Fe Outermost Electrons When we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that. Valence electrons are those in the outermost principal energy level. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Electrons in the outermost orbitals, called valence electrons, are responsible for most of the. Fe Outermost Electrons.
From www.slideshare.net
Structure Of Atoms Part 3 Fe Outermost Electrons As a result, when it gives up the two 4s electrons, it gains a valency of +2. In the periodic table, elements with analogous valence electron configurations usually occur within the same group. For example, sodium has one valence electron, and chlorine has seven valence electrons: Valence electrons are those in the outermost principal energy level. How to write the. Fe Outermost Electrons.
From www.slideserve.com
PPT Electrons PowerPoint Presentation, free download ID5118975 Fe Outermost Electrons As previously stated, iron has two valence states: Iron can also give up either of the paired electrons from a 3d. Valence electrons are those in the outermost principal energy level. In the periodic table, elements with analogous valence electron configurations usually occur within the same group. In layman’s terms, the number of electrons an element can receive, lose, or. Fe Outermost Electrons.