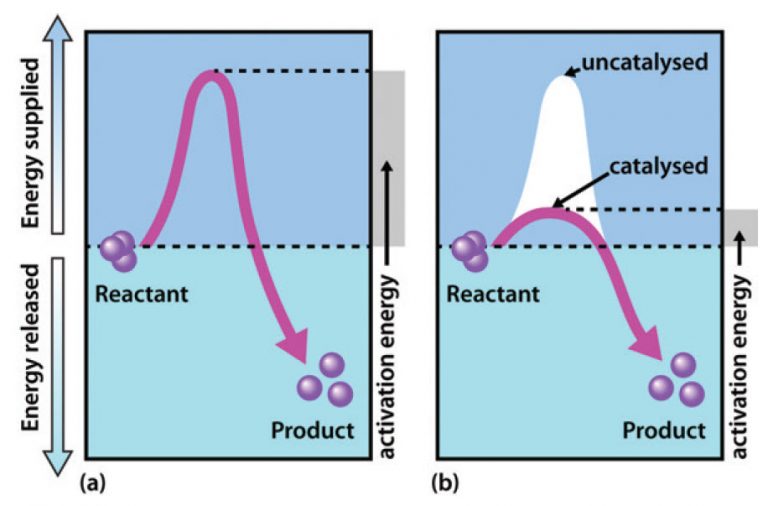
Catalyst Reaction Faster . The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without the. This page explains how adding a catalyst affects the rate of a reaction. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. When the reaction has finished, you would have exactly the same mass of. Catalysis is the process of speeding up a reaction using a catalyst. A catalyst does not actually become part of the products of. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. The chemical nature of the reacting substances, the physical state of the reactants, the temperature of the. We can identify five factors that affect the rates of chemical reactions:
from wou.edu
A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. This page explains how adding a catalyst affects the rate of a reaction. A catalyst does not actually become part of the products of. The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without the. We can identify five factors that affect the rates of chemical reactions: A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. The chemical nature of the reacting substances, the physical state of the reactants, the temperature of the. When the reaction has finished, you would have exactly the same mass of. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie.
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry
Catalyst Reaction Faster A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. When the reaction has finished, you would have exactly the same mass of. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without the. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. The chemical nature of the reacting substances, the physical state of the reactants, the temperature of the. Catalysis is the process of speeding up a reaction using a catalyst. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. We can identify five factors that affect the rates of chemical reactions: A catalyst does not actually become part of the products of. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. This page explains how adding a catalyst affects the rate of a reaction.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii Catalyst Reaction Faster A catalyst does not actually become part of the products of. Catalysis is the process of speeding up a reaction using a catalyst. When the reaction has finished, you would have exactly the same mass of. The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without the. This. Catalyst Reaction Faster.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalyst Reaction Faster Catalysis is the process of speeding up a reaction using a catalyst. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. This page explains how adding a catalyst affects the rate of a reaction. When the reaction has finished, you would have exactly the same mass of. The catalyst is manganese dioxide. Catalyst Reaction Faster.
From www.numerade.com
SOLVEDcatalyzed reactants products reaction progress Figure 2. The Catalyst Reaction Faster The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without the. A catalyst does not actually become part of the products of. We can identify five factors that affect the rates of chemical. Catalyst Reaction Faster.
From ppt-online.org
Rates of reaction презентация онлайн Catalyst Reaction Faster Catalysis is the process of speeding up a reaction using a catalyst. We can identify five factors that affect the rates of chemical reactions: A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end.. Catalyst Reaction Faster.
From www.youtube.com
Reaction Mechanisms and Rate Laws fast and slow first step YouTube Catalyst Reaction Faster A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. We can identify five factors that affect the rates of chemical reactions: A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. When the reaction has finished, you would have exactly. Catalyst Reaction Faster.
From www.youtube.com
How does a CATALYST work ? YouTube Catalyst Reaction Faster We can identify five factors that affect the rates of chemical reactions: A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without the. Catalysis is the process of. Catalyst Reaction Faster.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Reaction Faster A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. When the reaction has finished, you would have exactly the same mass of. The chemical nature of the reacting substances, the physical state of the reactants, the temperature. Catalyst Reaction Faster.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Reaction Faster A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. The chemical nature of the reacting substances, the physical state of the reactants, the temperature of the. A catalyst does not actually become part of the products of.. Catalyst Reaction Faster.
From socratic.org
Why does a catalyst cause a reaction to speed up? Socratic Catalyst Reaction Faster The chemical nature of the reacting substances, the physical state of the reactants, the temperature of the. The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without the. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction.. Catalyst Reaction Faster.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Reaction Faster A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. The chemical nature of the reacting substances, the physical state of the reactants, the temperature of the. Catalysis is the process of speeding up. Catalyst Reaction Faster.
From courses.lumenlearning.com
12.7 Catalysis General College Chemistry II Catalyst Reaction Faster We can identify five factors that affect the rates of chemical reactions: When the reaction has finished, you would have exactly the same mass of. This page explains how adding a catalyst affects the rate of a reaction. The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without. Catalyst Reaction Faster.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Reaction Faster When the reaction has finished, you would have exactly the same mass of. A catalyst does not actually become part of the products of. We can identify five factors that affect the rates of chemical reactions: A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. A catalyst is a substance. Catalyst Reaction Faster.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Catalyst Reaction Faster Catalysis is the process of speeding up a reaction using a catalyst. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. When the reaction has finished, you would have exactly the same mass. Catalyst Reaction Faster.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalyst Reaction Faster This page explains how adding a catalyst affects the rate of a reaction. The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without the. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. A catalyst does not actually become part. Catalyst Reaction Faster.
From www.youtube.com
Heterogeneous catalyst & catalysis 12th Std Chemistry Science Catalyst Reaction Faster The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without the. Catalysis is the process of speeding up a reaction using a catalyst. The chemical nature of the reacting substances, the physical state of the reactants, the temperature of the. A catalyst is a substance that can help. Catalyst Reaction Faster.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Reaction Faster We can identify five factors that affect the rates of chemical reactions: Catalysis is the process of speeding up a reaction using a catalyst. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. A catalyst. Catalyst Reaction Faster.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii Catalyst Reaction Faster This page explains how adding a catalyst affects the rate of a reaction. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. The catalyst is manganese dioxide and its presence causes the reaction shown above. Catalyst Reaction Faster.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Reaction Faster A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without the. The. Catalyst Reaction Faster.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Catalyst Reaction Faster This page explains how adding a catalyst affects the rate of a reaction. The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without the. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. Catalysis is the process. Catalyst Reaction Faster.
From study.com
Effect of Catalysts on Rates of Reaction Lesson Catalyst Reaction Faster A catalyst does not actually become part of the products of. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. The chemical nature of the reacting substances, the physical state of the reactants, the temperature of the. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end. Catalyst Reaction Faster.
From slideplayer.com
REACTION RATES Never mind making it go faster, what the hell is it we Catalyst Reaction Faster This page explains how adding a catalyst affects the rate of a reaction. The chemical nature of the reacting substances, the physical state of the reactants, the temperature of the. A catalyst does not actually become part of the products of. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. The catalyst. Catalyst Reaction Faster.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Catalyst Reaction Faster When the reaction has finished, you would have exactly the same mass of. Catalysis is the process of speeding up a reaction using a catalyst. The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without the. A catalyst is a substance which speeds up a reaction, but is. Catalyst Reaction Faster.
From www.slideserve.com
PPT RATES OF REACTION A guide for GCSE students PowerPoint Catalyst Reaction Faster We can identify five factors that affect the rates of chemical reactions: The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without the. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. A catalyst is a substance which speeds up a reaction,. Catalyst Reaction Faster.
From www.slideserve.com
PPT Mechanisms, Catalysts Intermediates and k PowerPoint Presentation Catalyst Reaction Faster This page explains how adding a catalyst affects the rate of a reaction. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. A catalyst does not actually become part of the products of. We can identify five factors that affect the rates of chemical reactions: The catalyst is manganese dioxide. Catalyst Reaction Faster.
From www.researchgate.net
Reaction time through using of Nano catalysts. Download Scientific Catalyst Reaction Faster A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. This page explains how adding a catalyst affects the rate of a reaction. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. A catalyst does not actually become part of. Catalyst Reaction Faster.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalyst Reaction Faster A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. When the reaction has finished, you would have exactly the same mass of. A catalyst does not actually become part of the products. Catalyst Reaction Faster.
From shapeguidance1.gitlab.io
Outrageous Does A Catalyst Increase The Rate Of Reaction Year 12 Catalyst Reaction Faster The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without the. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. A catalyst is a substance that can help the reactants in a chemical reaction react with each. Catalyst Reaction Faster.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalyst Reaction Faster The chemical nature of the reacting substances, the physical state of the reactants, the temperature of the. Catalysis is the process of speeding up a reaction using a catalyst. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. This page explains how adding a catalyst affects the rate of a reaction. A catalyst is. Catalyst Reaction Faster.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalyst Reaction Faster A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without the. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. A. Catalyst Reaction Faster.
From socratic.org
What will occur if a catalyst is added to a reaction mixture? Socratic Catalyst Reaction Faster The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysis is the process of speeding up a reaction using a catalyst. We can identify five factors that affect the rates of chemical reactions: A catalyst does not actually become part of the products of. When the reaction has finished, you would have exactly the. Catalyst Reaction Faster.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation ID591293 Catalyst Reaction Faster A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. When the reaction has finished, you would have exactly the same mass of. A catalyst does not actually become part of the products of. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. This. Catalyst Reaction Faster.
From www.youtube.com
Effect of Catalyst on Rate of Reaction YouTube Catalyst Reaction Faster The chemical nature of the reacting substances, the physical state of the reactants, the temperature of the. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysis is the process of speeding up a reaction. Catalyst Reaction Faster.
From www.numerade.com
SOLVED An uncatalyzed reaction has an activation energy of 60.0 kJ/mol Catalyst Reaction Faster The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysis is the process of speeding up a reaction using a catalyst. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. The chemical nature of the reacting substances, the physical state of the reactants, the temperature of. Catalyst Reaction Faster.
From slideplayer.com
REACTION RATES Never mind making it go faster, what the hell is it we Catalyst Reaction Faster The chemical nature of the reacting substances, the physical state of the reactants, the temperature of the. When the reaction has finished, you would have exactly the same mass of. We can identify five factors that affect the rates of chemical reactions: Catalysis is the process of speeding up a reaction using a catalyst. A catalyst is a substance which. Catalyst Reaction Faster.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Reaction Faster A catalyst does not actually become part of the products of. The catalyst is manganese dioxide and its presence causes the reaction shown above to run many times faster than it occurs without the. When the reaction has finished, you would have exactly the same mass of. A catalyst is a substance which speeds up a reaction, but is chemically. Catalyst Reaction Faster.