Titration Curve Worksheet Ap Chemistry . What would a graph of the slope of this curve. Whether the acid or base was. Initial ph must be > 7 (calculated ph ≈ 11) •2 nd pt. This is called a “titration curve.” questions: Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. There are four main points in a titration curve: A) the following table gives the result of slowly adding 1.000 m naoh to 1.000 l of 1.000 m hcl. The start where the solution only contains either acid or base. 1) this is a _________________(strong/weak) acid titrated with a strong base. The region where the titrant is added up to the equivalence point,. If the acids/bases are strong or weak. (b) sketch of titration curve: From the graph at the right, determine. At what point on this curve is the acid neutralized?
from general.chemistrysteps.com
What would a graph of the slope of this curve. From the graph at the right, determine. A) the following table gives the result of slowly adding 1.000 m naoh to 1.000 l of 1.000 m hcl. If the acids/bases are strong or weak. Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. There are four main points in a titration curve: Whether the acid or base was. At what point on this curve is the acid neutralized? The region where the titrant is added up to the equivalence point,. 1) this is a _________________(strong/weak) acid titrated with a strong base.
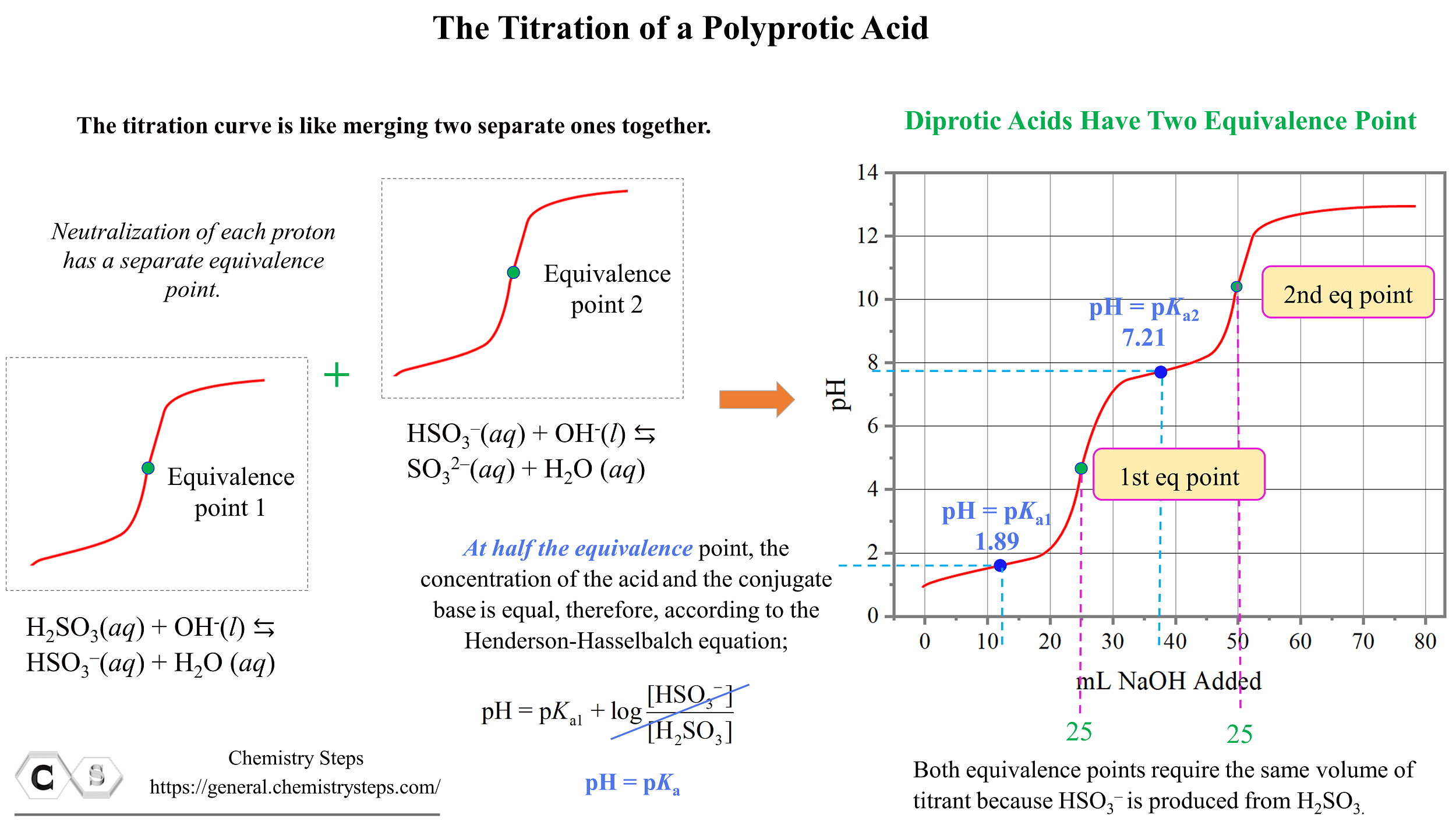
Titration of a Polyprotic Acids Chemistry Steps
Titration Curve Worksheet Ap Chemistry Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. 1) this is a _________________(strong/weak) acid titrated with a strong base. The start where the solution only contains either acid or base. What would a graph of the slope of this curve. Initial ph must be > 7 (calculated ph ≈ 11) •2 nd pt. Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. From the graph at the right, determine. At what point on this curve is the acid neutralized? This is called a “titration curve.” questions: Whether the acid or base was. If the acids/bases are strong or weak. There are four main points in a titration curve: (b) sketch of titration curve: The region where the titrant is added up to the equivalence point,. A) the following table gives the result of slowly adding 1.000 m naoh to 1.000 l of 1.000 m hcl.
From studylib.net
Titration Practice Worksheet Titration Curve Worksheet Ap Chemistry What would a graph of the slope of this curve. This is called a “titration curve.” questions: Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. The start where the solution only contains either acid or base. (b) sketch of titration curve: 1) this is a _________________(strong/weak) acid titrated with a strong base.. Titration Curve Worksheet Ap Chemistry.
From www.chegg.com
Solved Titration Of Strong And Weak Acids Smart Worksheet Titration Curve Worksheet Ap Chemistry The region where the titrant is added up to the equivalence point,. Initial ph must be > 7 (calculated ph ≈ 11) •2 nd pt. Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. Whether the acid or base was. There are four main points in a titration curve: 1) this is a. Titration Curve Worksheet Ap Chemistry.
From www.pinterest.com
Acid Base Titration Gcse Chemistry, Chemistry Notes, Ocr A Level Titration Curve Worksheet Ap Chemistry From the graph at the right, determine. Initial ph must be > 7 (calculated ph ≈ 11) •2 nd pt. This is called a “titration curve.” questions: The start where the solution only contains either acid or base. The region where the titrant is added up to the equivalence point,. 1) this is a _________________(strong/weak) acid titrated with a strong. Titration Curve Worksheet Ap Chemistry.
From education2research.com
Cracking the Code Unveiling the Titration Curves Worksheet Answers Titration Curve Worksheet Ap Chemistry From the graph at the right, determine. There are four main points in a titration curve: (b) sketch of titration curve: Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. If the acids/bases are strong or weak. Whether the acid or base was. A) the following table gives the result of slowly adding. Titration Curve Worksheet Ap Chemistry.
From mavink.com
Buffer Region Titration Curve Titration Curve Worksheet Ap Chemistry What would a graph of the slope of this curve. The start where the solution only contains either acid or base. Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. The region where the titrant is added up to the equivalence point,. Whether the acid or base was. At what point on this. Titration Curve Worksheet Ap Chemistry.
From www.learnsci.com
LearnSci Smart Worksheet Determine Concentration of HCl by Titration Titration Curve Worksheet Ap Chemistry If the acids/bases are strong or weak. At what point on this curve is the acid neutralized? Whether the acid or base was. (b) sketch of titration curve: Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. 1) this is a _________________(strong/weak) acid titrated with a strong base. From the graph at the. Titration Curve Worksheet Ap Chemistry.
From www.chegg.com
Solved 10. Use the lettered points on the titration curve to Titration Curve Worksheet Ap Chemistry 1) this is a _________________(strong/weak) acid titrated with a strong base. (b) sketch of titration curve: If the acids/bases are strong or weak. Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. What would a graph of the slope of this curve. The region where the titrant is added up to the equivalence. Titration Curve Worksheet Ap Chemistry.
From www.chemmybear.com
AP Chemistry Page Titration Curve Worksheet Ap Chemistry (b) sketch of titration curve: At what point on this curve is the acid neutralized? The region where the titrant is added up to the equivalence point,. Whether the acid or base was. Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. There are four main points in a titration curve: The start. Titration Curve Worksheet Ap Chemistry.
From chem.libretexts.org
11.3 Reaction Stoichiometry in Solutions AcidBase Titrations Titration Curve Worksheet Ap Chemistry If the acids/bases are strong or weak. A) the following table gives the result of slowly adding 1.000 m naoh to 1.000 l of 1.000 m hcl. Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. 1) this is a _________________(strong/weak) acid titrated with a strong base. Initial ph must be > 7. Titration Curve Worksheet Ap Chemistry.
From ar.inspiredpencil.com
Titration Curve Labeled Titration Curve Worksheet Ap Chemistry The start where the solution only contains either acid or base. If the acids/bases are strong or weak. 1) this is a _________________(strong/weak) acid titrated with a strong base. A) the following table gives the result of slowly adding 1.000 m naoh to 1.000 l of 1.000 m hcl. This is called a “titration curve.” questions: Whether the acid or. Titration Curve Worksheet Ap Chemistry.
From proofreadingacademic.web.fc2.com
Write my Paper for Cheap in High Quality acid base titrations lab Titration Curve Worksheet Ap Chemistry Whether the acid or base was. At what point on this curve is the acid neutralized? The start where the solution only contains either acid or base. A) the following table gives the result of slowly adding 1.000 m naoh to 1.000 l of 1.000 m hcl. From the graph at the right, determine. 1) this is a _________________(strong/weak) acid. Titration Curve Worksheet Ap Chemistry.
From www.purechemistry.org
ACIDBASE TITRATION Purechemistry Titration Curve Worksheet Ap Chemistry (b) sketch of titration curve: 1) this is a _________________(strong/weak) acid titrated with a strong base. Initial ph must be > 7 (calculated ph ≈ 11) •2 nd pt. The start where the solution only contains either acid or base. The region where the titrant is added up to the equivalence point,. A) the following table gives the result of. Titration Curve Worksheet Ap Chemistry.
From worksheets.clipart-library.com
Solved Worksheet (8.4) AcidBase Titration Curve A student Titration Curve Worksheet Ap Chemistry A) the following table gives the result of slowly adding 1.000 m naoh to 1.000 l of 1.000 m hcl. (b) sketch of titration curve: Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. What would a graph of the slope of this curve. From the graph at the right, determine. This is. Titration Curve Worksheet Ap Chemistry.
From www.albert.io
[HF] and [F^] Comparison from a Titration Curve AP® Chemistry Titration Curve Worksheet Ap Chemistry 1) this is a _________________(strong/weak) acid titrated with a strong base. Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. The start where the solution only contains either acid or base. What would a graph of the slope of this curve. Initial ph must be > 7 (calculated ph ≈ 11) •2 nd. Titration Curve Worksheet Ap Chemistry.
From www.chegg.com
Solved Titration Curves Worksheet The following are Weak Titration Curve Worksheet Ap Chemistry A) the following table gives the result of slowly adding 1.000 m naoh to 1.000 l of 1.000 m hcl. There are four main points in a titration curve: At what point on this curve is the acid neutralized? If the acids/bases are strong or weak. This is called a “titration curve.” questions: Equivalence point occurs at 15.0 ml ±. Titration Curve Worksheet Ap Chemistry.
From www.chemistryworksheet.com
Chemistry Titration Worksheet Answers Titration Curve Worksheet Ap Chemistry What would a graph of the slope of this curve. 1) this is a _________________(strong/weak) acid titrated with a strong base. If the acids/bases are strong or weak. (b) sketch of titration curve: The start where the solution only contains either acid or base. Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be.. Titration Curve Worksheet Ap Chemistry.
From www.showme.com
ap chemistry weak acid/strong base titration curves Science Titration Curve Worksheet Ap Chemistry From the graph at the right, determine. Whether the acid or base was. Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. The region where the titrant is added up to the equivalence point,. At what point on this curve is the acid neutralized? What would a graph of the slope of this. Titration Curve Worksheet Ap Chemistry.
From www.studypool.com
SOLUTION Titration Curve 2 Studypool Titration Curve Worksheet Ap Chemistry At what point on this curve is the acid neutralized? From the graph at the right, determine. If the acids/bases are strong or weak. What would a graph of the slope of this curve. 1) this is a _________________(strong/weak) acid titrated with a strong base. The region where the titrant is added up to the equivalence point,. (b) sketch of. Titration Curve Worksheet Ap Chemistry.
From www.studocu.com
1051 Exp 8 Titration Curve F2023 Chemistry 1051 Laboratory Chemistry Titration Curve Worksheet Ap Chemistry A) the following table gives the result of slowly adding 1.000 m naoh to 1.000 l of 1.000 m hcl. Initial ph must be > 7 (calculated ph ≈ 11) •2 nd pt. This is called a “titration curve.” questions: The region where the titrant is added up to the equivalence point,. There are four main points in a titration. Titration Curve Worksheet Ap Chemistry.
From www.savemyexams.com
pH Titration Curves OCR A Level Chemistry Revision Notes 2017 Titration Curve Worksheet Ap Chemistry This is called a “titration curve.” questions: If the acids/bases are strong or weak. Initial ph must be > 7 (calculated ph ≈ 11) •2 nd pt. Whether the acid or base was. Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. There are four main points in a titration curve: The region. Titration Curve Worksheet Ap Chemistry.
From www.animalia-life.club
Titration Curve Amino Acid Titration Curve Worksheet Ap Chemistry At what point on this curve is the acid neutralized? (b) sketch of titration curve: The start where the solution only contains either acid or base. From the graph at the right, determine. Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. A) the following table gives the result of slowly adding 1.000. Titration Curve Worksheet Ap Chemistry.
From www.chemistrylearner.com
Free Printable Acids and Bases Titration Worksheets Titration Curve Worksheet Ap Chemistry 1) this is a _________________(strong/weak) acid titrated with a strong base. From the graph at the right, determine. (b) sketch of titration curve: Whether the acid or base was. A) the following table gives the result of slowly adding 1.000 m naoh to 1.000 l of 1.000 m hcl. Equivalence point occurs at 15.0 ml ± 1 ml of hcl. Titration Curve Worksheet Ap Chemistry.
From quizlet.com
Weak Acid Strong Base Titration Curve Particle View Diagram Quizlet Titration Curve Worksheet Ap Chemistry A) the following table gives the result of slowly adding 1.000 m naoh to 1.000 l of 1.000 m hcl. There are four main points in a titration curve: If the acids/bases are strong or weak. Initial ph must be > 7 (calculated ph ≈ 11) •2 nd pt. From the graph at the right, determine. At what point on. Titration Curve Worksheet Ap Chemistry.
From education2research.com
Cracking the Code Unveiling the Titration Curves Worksheet Answers Titration Curve Worksheet Ap Chemistry What would a graph of the slope of this curve. 1) this is a _________________(strong/weak) acid titrated with a strong base. At what point on this curve is the acid neutralized? Whether the acid or base was. (b) sketch of titration curve: Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. A) the. Titration Curve Worksheet Ap Chemistry.
From chem.libretexts.org
11.3 Reaction Stoichiometry in Solutions AcidBase Titrations Titration Curve Worksheet Ap Chemistry This is called a “titration curve.” questions: 1) this is a _________________(strong/weak) acid titrated with a strong base. The region where the titrant is added up to the equivalence point,. If the acids/bases are strong or weak. (b) sketch of titration curve: Whether the acid or base was. There are four main points in a titration curve: At what point. Titration Curve Worksheet Ap Chemistry.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.7.12 pH Titration Curves翰林国际教育 Titration Curve Worksheet Ap Chemistry The start where the solution only contains either acid or base. 1) this is a _________________(strong/weak) acid titrated with a strong base. This is called a “titration curve.” questions: Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. Whether the acid or base was. Initial ph must be > 7 (calculated ph ≈. Titration Curve Worksheet Ap Chemistry.
From www.youtube.com
AP Chem unit 8.5 Titration Curves YouTube Titration Curve Worksheet Ap Chemistry A) the following table gives the result of slowly adding 1.000 m naoh to 1.000 l of 1.000 m hcl. Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. At what point on this curve is the acid neutralized? Initial ph must be > 7 (calculated ph ≈ 11) •2 nd pt. What. Titration Curve Worksheet Ap Chemistry.
From ar.inspiredpencil.com
Titration Curve Labeled Titration Curve Worksheet Ap Chemistry What would a graph of the slope of this curve. Whether the acid or base was. This is called a “titration curve.” questions: If the acids/bases are strong or weak. From the graph at the right, determine. Initial ph must be > 7 (calculated ph ≈ 11) •2 nd pt. There are four main points in a titration curve: Equivalence. Titration Curve Worksheet Ap Chemistry.
From www.vrogue.co
Titration Worksheet Key Thekidsworksheet vrogue.co Titration Curve Worksheet Ap Chemistry From the graph at the right, determine. This is called a “titration curve.” questions: The start where the solution only contains either acid or base. If the acids/bases are strong or weak. There are four main points in a titration curve: Initial ph must be > 7 (calculated ph ≈ 11) •2 nd pt. Equivalence point occurs at 15.0 ml. Titration Curve Worksheet Ap Chemistry.
From www.youtube.com
Drawing titration curves for amino acids strategy, intuition, and Titration Curve Worksheet Ap Chemistry From the graph at the right, determine. Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. A) the following table gives the result of slowly adding 1.000 m naoh to 1.000 l of 1.000 m hcl. What would a graph of the slope of this curve. 1) this is a _________________(strong/weak) acid titrated. Titration Curve Worksheet Ap Chemistry.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Worksheet Ap Chemistry (b) sketch of titration curve: Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. From the graph at the right, determine. If the acids/bases are strong or weak. The region where the titrant is added up to the equivalence point,. What would a graph of the slope of this curve. This is called. Titration Curve Worksheet Ap Chemistry.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Curve Worksheet Ap Chemistry Whether the acid or base was. From the graph at the right, determine. A) the following table gives the result of slowly adding 1.000 m naoh to 1.000 l of 1.000 m hcl. The start where the solution only contains either acid or base. Initial ph must be > 7 (calculated ph ≈ 11) •2 nd pt. (b) sketch of. Titration Curve Worksheet Ap Chemistry.
From ar.inspiredpencil.com
Titration Curve Labeled Titration Curve Worksheet Ap Chemistry What would a graph of the slope of this curve. 1) this is a _________________(strong/weak) acid titrated with a strong base. If the acids/bases are strong or weak. (b) sketch of titration curve: Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. Whether the acid or base was. At what point on this. Titration Curve Worksheet Ap Chemistry.
From www.youtube.com
AP Chemistry Titration Graph problem worksheet review YouTube Titration Curve Worksheet Ap Chemistry If the acids/bases are strong or weak. 1) this is a _________________(strong/weak) acid titrated with a strong base. Equivalence point occurs at 15.0 ml ± 1 ml of hcl added (equivalence point must be. A) the following table gives the result of slowly adding 1.000 m naoh to 1.000 l of 1.000 m hcl. This is called a “titration curve.”. Titration Curve Worksheet Ap Chemistry.
From www.studocu.com
Titration Curve Practice Problems MATH + SCIENCE INITIATIVE Titration Titration Curve Worksheet Ap Chemistry From the graph at the right, determine. 1) this is a _________________(strong/weak) acid titrated with a strong base. Initial ph must be > 7 (calculated ph ≈ 11) •2 nd pt. The region where the titrant is added up to the equivalence point,. Whether the acid or base was. What would a graph of the slope of this curve. A). Titration Curve Worksheet Ap Chemistry.