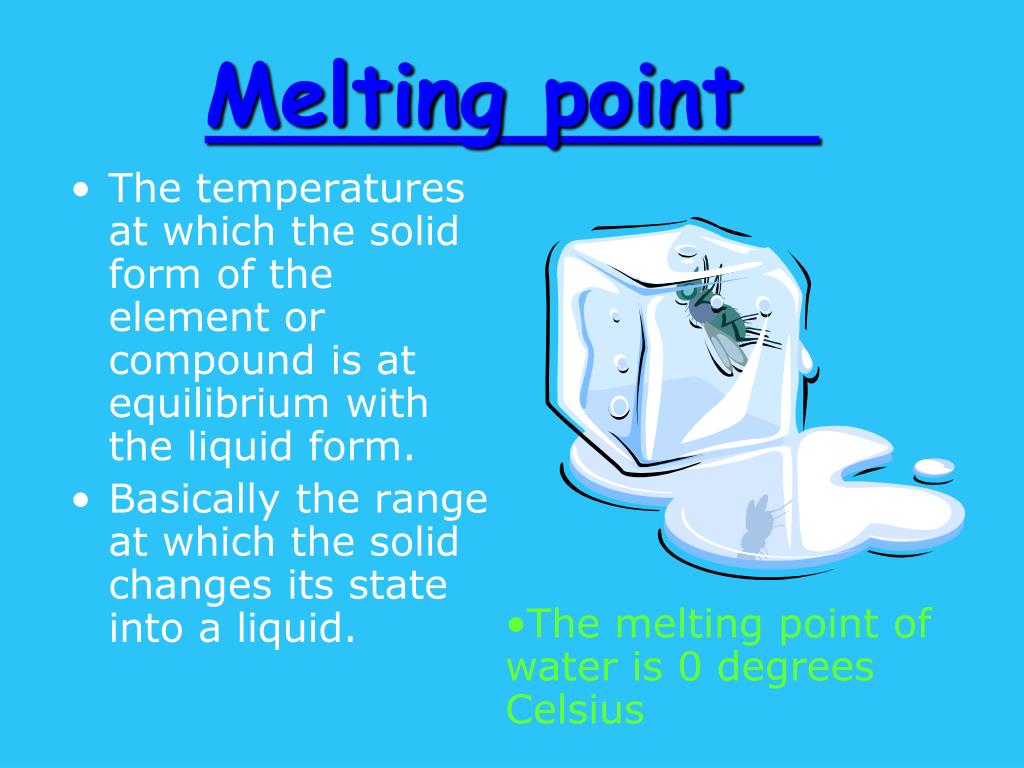
Boiling Point Definition In Chemistry . — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. — boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. learn what boiling point is and how it depends on external pressure. See how altitude affects the boiling point of water. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \:
from www.slideserve.com
— boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. learn what boiling point is and how it depends on external pressure. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. See how altitude affects the boiling point of water.
PPT Physical and Chemical Properties PowerPoint Presentation, free download ID2756402
Boiling Point Definition In Chemistry learn what boiling point is and how it depends on external pressure. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. — boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. See how altitude affects the boiling point of water. learn what boiling point is and how it depends on external pressure. — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point.
From schools.aglasem.com
CBSE Class 12 Chemistry Notes Solutions Elevation in Boiling Point Boiling Point Definition In Chemistry — boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \:. Boiling Point Definition In Chemistry.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Boiling Point Definition In Chemistry See how altitude affects the boiling point of water. learn what boiling point is and how it depends on external pressure. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted.. Boiling Point Definition In Chemistry.
From chemistnotes.com
Boiling Point Definition, Boiling point as physical properties Chemistry Notes Boiling Point Definition In Chemistry learn what boiling point is and how it depends on external pressure. — boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: boiling point, temperature at which the pressure. Boiling Point Definition In Chemistry.
From www.slideserve.com
PPT ORGANIC CHEMISTRY PowerPoint Presentation, free download ID1562148 Boiling Point Definition In Chemistry — boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. See how altitude affects the boiling point of water. — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. a compound's normal boiling point refers to its. Boiling Point Definition In Chemistry.
From www.slideserve.com
PPT Chemical Properties PowerPoint Presentation, free download ID2262680 Boiling Point Definition In Chemistry — boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. See how altitude affects the boiling point of water. — if this pressure is the standard. Boiling Point Definition In Chemistry.
From www.slideserve.com
PPT Physical And Chemical Properties PowerPoint Presentation, free download ID2048481 Boiling Point Definition In Chemistry boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. — the boiling point is the temperature at. Boiling Point Definition In Chemistry.
From matmake.com
Boiling Point Definition, Measurements, and Applications Boiling Point Definition In Chemistry — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. a compound's normal boiling point refers to its boiling. Boiling Point Definition In Chemistry.
From ppt-online.org
theory of ideal gases презентация онлайн Boiling Point Definition In Chemistry a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: See how altitude affects the boiling point of water. learn what boiling point is and how it depends on external pressure. — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. Boiling Point Definition In Chemistry.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin Boiling Point Definition In Chemistry — boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. learn what boiling point is and how it depends on external pressure. See how altitude affects the boiling point of water. — the boiling point is the temperature at which the vapor pressure of a liquid. Boiling Point Definition In Chemistry.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Boiling Point Definition In Chemistry — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. See how altitude affects the boiling point of water. — if this pressure is the standard pressure of. Boiling Point Definition In Chemistry.
From chemhero.blogspot.com
Definition Of Boiling In Chemistry Chem Hero Boiling Point Definition In Chemistry a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: learn what boiling point is and how it depends on external pressure. — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point.. Boiling Point Definition In Chemistry.
From facts.net
20 Fascinating Facts About Boiling Point Elevation Boiling Point Definition In Chemistry a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. — the boiling point is the temperature at which the vapor pressure. Boiling Point Definition In Chemistry.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Boiling Point Definition In Chemistry — boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature. Boiling Point Definition In Chemistry.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Boiling Point Definition In Chemistry See how altitude affects the boiling point of water. — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. learn what boiling point is and how it depends on external pressure. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by. Boiling Point Definition In Chemistry.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Boiling Point Definition In Chemistry — boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by. Boiling Point Definition In Chemistry.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills Boiling Point Definition In Chemistry — boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. — the boiling point is the temperature at which the vapor pressure of a liquid equals. Boiling Point Definition In Chemistry.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition, Conditions, Characteristics Boiling Point Definition In Chemistry See how altitude affects the boiling point of water. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: — boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. — if this pressure is the standard pressure of 1 atm. Boiling Point Definition In Chemistry.
From www.youtube.com
AP Chemistry multiple choice sample Boiling points YouTube Boiling Point Definition In Chemistry — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. learn what boiling point is and how it depends on external pressure. — the boiling point is the temperature at which the vapor pressure of a liquid equals. Boiling Point Definition In Chemistry.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Boiling Point Definition In Chemistry boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils. Boiling Point Definition In Chemistry.
From www.slideserve.com
PPT Physical and Chemical Properties PowerPoint Presentation, free download ID2756402 Boiling Point Definition In Chemistry a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. — boiling is the process by which a liquid turns into a. Boiling Point Definition In Chemistry.
From www.healthbenefitstimes.com
Boiling Point Definition of Boiling Point Boiling Point Definition In Chemistry — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. learn what boiling point is and how it depends. Boiling Point Definition In Chemistry.
From jsmithmoore.com
Boiling point of ethanol celsius Boiling Point Definition In Chemistry — boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. — the boiling point is the temperature at which the vapor pressure of a liquid equals. Boiling Point Definition In Chemistry.
From www.thoughtco.com
Definition of Boiling Point in Chemistry Boiling Point Definition In Chemistry learn what boiling point is and how it depends on external pressure. See how altitude affects the boiling point of water. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted.. Boiling Point Definition In Chemistry.
From chemistnotes.com
Elevation of Boiling Point Definition/Equation/Molal Boiling Point Elevation Chemistry Notes Boiling Point Definition In Chemistry — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. a compound's normal boiling point refers to its boiling. Boiling Point Definition In Chemistry.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Boiling Point Definition In Chemistry learn what boiling point is and how it depends on external pressure. — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. a compound's normal boiling point. Boiling Point Definition In Chemistry.
From www.thoughtco.com
Normal Boiling Point Definition (Chemistry) Boiling Point Definition In Chemistry boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. See how altitude affects the boiling point of water. — boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. — the boiling point is the temperature. Boiling Point Definition In Chemistry.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Boiling Point Definition In Chemistry — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. learn what boiling point is and how it depends on external pressure. See how altitude affects the boiling. Boiling Point Definition In Chemistry.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Boiling Point Definition In Chemistry See how altitude affects the boiling point of water. — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted.. Boiling Point Definition In Chemistry.
From www.slideserve.com
PPT Flash PointIgnition Point PowerPoint Presentation ID310797 Boiling Point Definition In Chemistry — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: — the boiling point is the temperature at which the vapor pressure. Boiling Point Definition In Chemistry.
From www.worksheetsplanet.com
What is Boiling Point Boiling Point Definition In Chemistry learn what boiling point is and how it depends on external pressure. — boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to. Boiling Point Definition In Chemistry.
From gamesmartz.com
Boiling Point Elevation Definition & Image GameSmartz Boiling Point Definition In Chemistry a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. learn. Boiling Point Definition In Chemistry.
From guidemanualeruptivity.z14.web.core.windows.net
Normal Boiling Point Chemistry Boiling Point Definition In Chemistry — if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. — boiling is the process by which a liquid. Boiling Point Definition In Chemistry.
From www.youtube.com
Boiling Point of Organic Compounds YouTube Boiling Point Definition In Chemistry learn what boiling point is and how it depends on external pressure. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. boiling point, temperature at which the pressure exerted by. Boiling Point Definition In Chemistry.
From definitionklw.blogspot.com
Boiling Point Definition Chemistry DEFINITION KLW Boiling Point Definition In Chemistry learn what boiling point is and how it depends on external pressure. — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. a compound's normal boiling point. Boiling Point Definition In Chemistry.
From www.slideserve.com
PPT Periodic Table Trends Melting & Boiling Point PowerPoint Presentation ID3047649 Boiling Point Definition In Chemistry — the boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: learn what boiling point is and how it depends on external pressure. — boiling is the process by which a liquid. Boiling Point Definition In Chemistry.