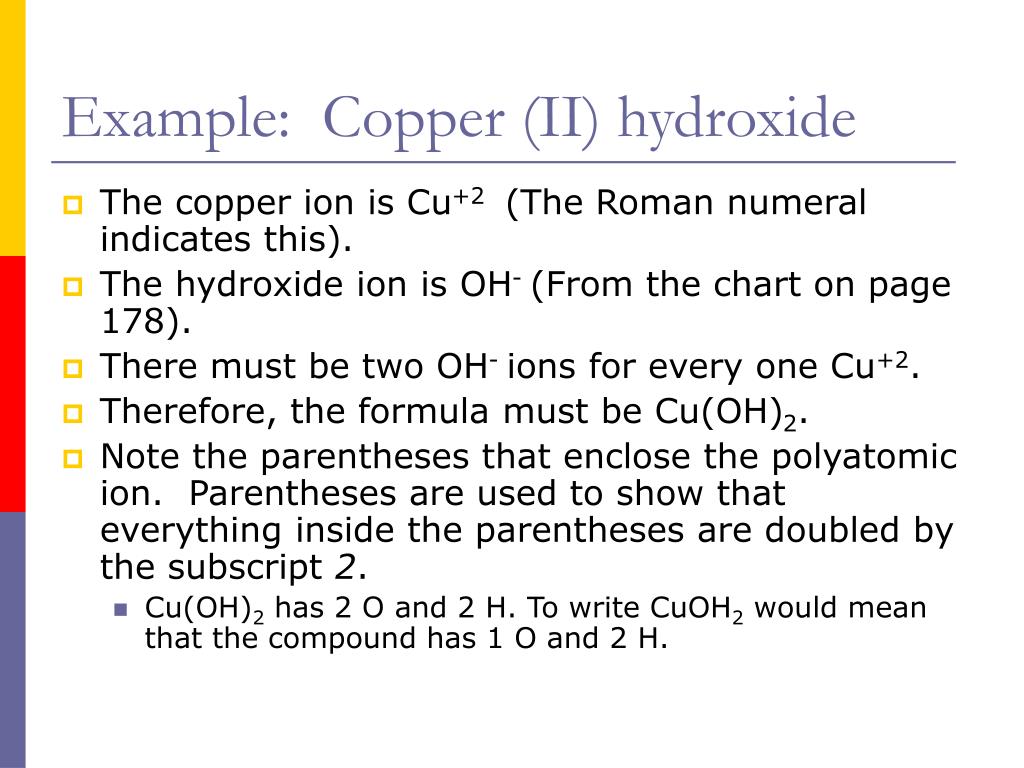
Copper (Ii) Hydroxide + Hcl . the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper (ii) oxide. Copper(ii) hydroxide as a basic oxide; there are three main steps for writing the net ionic equation for cu (oh)2 +. This page describes and explains the. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. chromium(iii) hydroxide as an amphoteric hydroxide; Cucl2 is quite soluble in water: Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen. reactions of hexaaquacopper (ii) ions with hydroxide ions.
from www.slideserve.com
the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. Copper(ii) hydroxide as a basic oxide; Cucl2 is quite soluble in water: This page describes and explains the. there are three main steps for writing the net ionic equation for cu (oh)2 +. chromium(iii) hydroxide as an amphoteric hydroxide; this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper (ii) oxide. reactions of hexaaquacopper (ii) ions with hydroxide ions.
PPT Polyatomic Ions and Their Compounds PowerPoint Presentation ID
Copper (Ii) Hydroxide + Hcl if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. reactions of hexaaquacopper (ii) ions with hydroxide ions. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper (ii) oxide. Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. there are three main steps for writing the net ionic equation for cu (oh)2 +. Cucl2 is quite soluble in water: This page describes and explains the. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. chromium(iii) hydroxide as an amphoteric hydroxide; the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Copper(ii) hydroxide as a basic oxide; Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen. if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions.
From www.slideserve.com
PPT Polyatomic Ions and Their Compounds PowerPoint Presentation ID Copper (Ii) Hydroxide + Hcl the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. Cucl2 is quite soluble in water: this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper (ii) oxide. chromium(iii) hydroxide as an amphoteric hydroxide;. Copper (Ii) Hydroxide + Hcl.
From www.numerade.com
SOLVED A precipitate of copper(II) hydroxide is formed in a reaction Copper (Ii) Hydroxide + Hcl chromium(iii) hydroxide as an amphoteric hydroxide; if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. Copper(ii) hydroxide as a basic oxide; the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. there are three. Copper (Ii) Hydroxide + Hcl.
From www.alibaba.com
Copper(ii) Hydroxide Fungicide 97tc 77wp Buy Copper(ii) Hydroxide Copper (Ii) Hydroxide + Hcl there are three main steps for writing the net ionic equation for cu (oh)2 +. reactions of hexaaquacopper (ii) ions with hydroxide ions. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. this reaction is a type of double replacement reaction called a neutralization reaction, in which. Copper (Ii) Hydroxide + Hcl.
From www.chegg.com
Solved 3. Copper (II) hydroxide is amphoteric. What would Copper (Ii) Hydroxide + Hcl copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper (ii) oxide. if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions.. Copper (Ii) Hydroxide + Hcl.
From vanittaran.weebly.com
Synthesis of copper(II) hydroxide ACQUIRE Copper (Ii) Hydroxide + Hcl Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. chromium(iii) hydroxide as an amphoteric hydroxide; copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. This page describes and explains the. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen. there are three main steps for writing the net ionic equation for cu (oh)2 +.. Copper (Ii) Hydroxide + Hcl.
From www.docdroid.net
Copper(II) hydroxide SA.pdf DocDroid Copper (Ii) Hydroxide + Hcl there are three main steps for writing the net ionic equation for cu (oh)2 +. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Cucl2 is quite soluble in water: Copper(ii) hydroxide as a. Copper (Ii) Hydroxide + Hcl.
From www.youtube.com
Reduction of the copper(II) hydroxide by glucose in the alkaline medium Copper (Ii) Hydroxide + Hcl chromium(iii) hydroxide as an amphoteric hydroxide; if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. there are three main steps for writing the net ionic equation for cu (oh)2 +. Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. this reaction is a type. Copper (Ii) Hydroxide + Hcl.
From www.ceramic-glazes.com
Copper Hydroxide Cupric hydroxide patina for ceramics Copper (Ii) Hydroxide + Hcl the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Copper(ii) hydroxide as a basic oxide; Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen. Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper. Copper (Ii) Hydroxide + Hcl.
From www.flickr.com
Copper hydroxide Copper hydroxide (Cu(OH)2) suspended in s… Flickr Copper (Ii) Hydroxide + Hcl Cucl2 is quite soluble in water: Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen. if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. Copper(ii) hydroxide as a basic oxide; Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. this reaction is a type of double. Copper (Ii) Hydroxide + Hcl.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper (Ii) Hydroxide + Hcl copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. chromium(iii) hydroxide as an amphoteric hydroxide; Cucl2 is quite soluble in water: there are three main steps for writing the net ionic equation for cu (oh)2 +. This page describes and explains the. Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. this reaction. Copper (Ii) Hydroxide + Hcl.
From handwiki.org
ChemistryCopper(II) hydroxide HandWiki Copper (Ii) Hydroxide + Hcl Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. Cucl2 is quite soluble in water: if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. reactions of hexaaquacopper. Copper (Ii) Hydroxide + Hcl.
From thanyasuwankhajit.weebly.com
Lab Report for Copper (II) Hydroxide Synthesis EPORTFOLIO Copper (Ii) Hydroxide + Hcl if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. Copper(ii) hydroxide as a basic oxide; Cucl2 is quite soluble in water: This page describes and explains the. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. Copper (Ii) Hydroxide + Hcl.
From www.youtube.com
[Thí nghiệm số 4] CuSO4 + NaOH, Cu(OH)2 + HCl (copper (II) sulfate Copper (Ii) Hydroxide + Hcl copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. there are three main steps for writing the net ionic equation for cu (oh)2 +. chromium(iii) hydroxide as an amphoteric hydroxide; Cucl2 is quite soluble in water: if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six. Copper (Ii) Hydroxide + Hcl.
From www.dreamstime.com
Copper(II) Sulfate with Molecular Structure. Chemical Ingredient Used Copper (Ii) Hydroxide + Hcl there are three main steps for writing the net ionic equation for cu (oh)2 +. Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light. Copper (Ii) Hydroxide + Hcl.
From www.pdfprof.com
PDF copper hydroxide PDF Télécharger Download Copper (Ii) Hydroxide + Hcl Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen. chromium(iii) hydroxide as an amphoteric hydroxide; Copper(ii) hydroxide as a basic oxide; if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. there are three main steps for writing the net ionic equation for cu. Copper (Ii) Hydroxide + Hcl.
From www.youtube.com
Type of Reaction for Cu + HCl (Copper + Hydrochloric acid) YouTube Copper (Ii) Hydroxide + Hcl if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. This page describes and explains the. chromium(iii) hydroxide as an amphoteric hydroxide; there are three main steps for writing the net ionic equation for cu (oh)2 +. Copper(ii) hydroxide as a basic oxide; Hydroxide. Copper (Ii) Hydroxide + Hcl.
From www.numerade.com
SOLVED 6. Copper(II) oxide reacts with hydrochloric acid to produce Copper (Ii) Hydroxide + Hcl this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper (ii) oxide. chromium(iii) hydroxide as an amphoteric hydroxide; reactions of hexaaquacopper (ii) ions with hydroxide ions. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue. Copper (Ii) Hydroxide + Hcl.
From chaugiang.net.vn
Copper hydroxide CÔNG TY TNHH THIẾT BỊ VÀ CÔNG NGHỆ CHÂU GIANG Copper (Ii) Hydroxide + Hcl if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. Cucl2 is quite soluble in water: reactions of hexaaquacopper (ii) ions with hydroxide ions. chromium(iii) hydroxide as an amphoteric hydroxide; there are three main steps for writing the net ionic equation for cu. Copper (Ii) Hydroxide + Hcl.
From www.alamy.com
Copper(II) sulfate and Sodium Hydroxide Pellets in test tube with plug Copper (Ii) Hydroxide + Hcl Cucl2 is quite soluble in water: if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. reactions of hexaaquacopper (ii) ions with hydroxide ions. chromium(iii) hydroxide as an amphoteric hydroxide; the production of copper (ii) hydroxide typically occurs through the reaction of copper. Copper (Ii) Hydroxide + Hcl.
From ceeqicat.blob.core.windows.net
Copper 2 Hydroxide Formula at Ryan Strader blog Copper (Ii) Hydroxide + Hcl This page describes and explains the. Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. reactions of hexaaquacopper (ii) ions with hydroxide ions. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper (ii) oxide. chromium(iii) hydroxide as an amphoteric hydroxide; if you add concentrated hydrochloric acid to a solution. Copper (Ii) Hydroxide + Hcl.
From oricoglobal.en.made-in-china.com
Copper (II) Hydroxide 97TC 77WP Fungicide China Copper Hydroxide 77 Copper (Ii) Hydroxide + Hcl if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. reactions of hexaaquacopper (ii) ions with hydroxide ions. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper (ii) oxide. Cu(oh)2(s) +2h cl(aq) → cucl2(aq) +. Copper (Ii) Hydroxide + Hcl.
From testbook.com
Copper Hydroxide Learn Definition, Structure, Formula, Uses here Copper (Ii) Hydroxide + Hcl chromium(iii) hydroxide as an amphoteric hydroxide; This page describes and explains the. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper (ii) oxide. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen. Copper(ii) hydroxide as a basic oxide; the production of copper (ii) hydroxide typically occurs through the. Copper (Ii) Hydroxide + Hcl.
From www.youtube.com
Copper(II) hydroxide (Cu(OH)2) precipitation YouTube Copper (Ii) Hydroxide + Hcl chromium(iii) hydroxide as an amphoteric hydroxide; there are three main steps for writing the net ionic equation for cu (oh)2 +. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Copper(ii) hydroxide as a basic oxide; Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. this reaction is a type of double replacement. Copper (Ii) Hydroxide + Hcl.
From www.sciencephoto.com
Copper hydroxide precipitate Stock Image A500/0411 Science Photo Copper (Ii) Hydroxide + Hcl copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen. reactions of hexaaquacopper (ii) ions with hydroxide ions. Copper(ii) hydroxide as. Copper (Ii) Hydroxide + Hcl.
From thanyasuwankhajit.weebly.com
Lab Report for Copper (II) Hydroxide Synthesis EPORTFOLIO Copper (Ii) Hydroxide + Hcl Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. Copper(ii) hydroxide as a basic oxide; Cucl2 is quite soluble in water: copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Hydroxide ions. Copper (Ii) Hydroxide + Hcl.
From www.youtube.com
Making copper hydroxide YouTube Copper (Ii) Hydroxide + Hcl Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen. This page describes and explains the. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Copper(ii) hydroxide as a basic oxide; if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced. Copper (Ii) Hydroxide + Hcl.
From www.docdroid.net
Copper(II) hydroxide SA.pdf DocDroid Copper (Ii) Hydroxide + Hcl reactions of hexaaquacopper (ii) ions with hydroxide ions. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Cucl2 is quite soluble in water: This page describes and explains the. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper (ii) oxide. the production. Copper (Ii) Hydroxide + Hcl.
From www.fishersci.com
Copper(II) hydroxide, tech. 94, stab., Alfa Aesar Fisher Scientific Copper (Ii) Hydroxide + Hcl Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Copper(ii) hydroxide as a basic oxide; Hydroxide ions (from, say,. Copper (Ii) Hydroxide + Hcl.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Copper (Ii) Hydroxide + Hcl Cucl2 is quite soluble in water: Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen. Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper (ii) oxide. This page describes and explains the. there are three main steps for writing the net ionic. Copper (Ii) Hydroxide + Hcl.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper (Ii) Hydroxide + Hcl if you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. there are three main steps for writing the net ionic equation for cu (oh)2 +. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to. Copper (Ii) Hydroxide + Hcl.
From www.youtube.com
Test for Copper (II) ion with Sodium Hydroxide YouTube Copper (Ii) Hydroxide + Hcl Copper(ii) hydroxide as a basic oxide; the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. chromium(iii) hydroxide as an amphoteric hydroxide; reactions of hexaaquacopper (ii) ions with hydroxide ions. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen. if you add concentrated hydrochloric acid to a solution. Copper (Ii) Hydroxide + Hcl.
From www.pc-chem.com
Copper(II) hydroxide Copper (Ii) Hydroxide + Hcl copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper (ii) oxide. there are three main steps for writing the net ionic equation for cu (oh)2 +. Cucl2 is quite soluble in water: the. Copper (Ii) Hydroxide + Hcl.
From dexatama.co.id
Copper(II) hydroxide Dexatama.co.id Copper (Ii) Hydroxide + Hcl chromium(iii) hydroxide as an amphoteric hydroxide; Cucl2 is quite soluble in water: this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper (ii) oxide. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Copper(ii) hydroxide as a basic oxide; Hydroxide ions (from, say, sodium. Copper (Ii) Hydroxide + Hcl.
From www.youtube.com
Tris(ethylenediamine) copper(II) sulfate monohydrate synthesis YouTube Copper (Ii) Hydroxide + Hcl Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. reactions of hexaaquacopper (ii) ions with hydroxide ions. Cucl2 is quite soluble in water: Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia. Copper (Ii) Hydroxide + Hcl.
From www.limac.lv
Oxides / Hydroxides Catalog LiMac Science Copper (Ii) Hydroxide + Hcl this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper (ii) oxide. Cu(oh)2(s) +2h cl(aq) → cucl2(aq) + 2h. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. chromium(iii) hydroxide as an amphoteric hydroxide; there are three main steps. Copper (Ii) Hydroxide + Hcl.