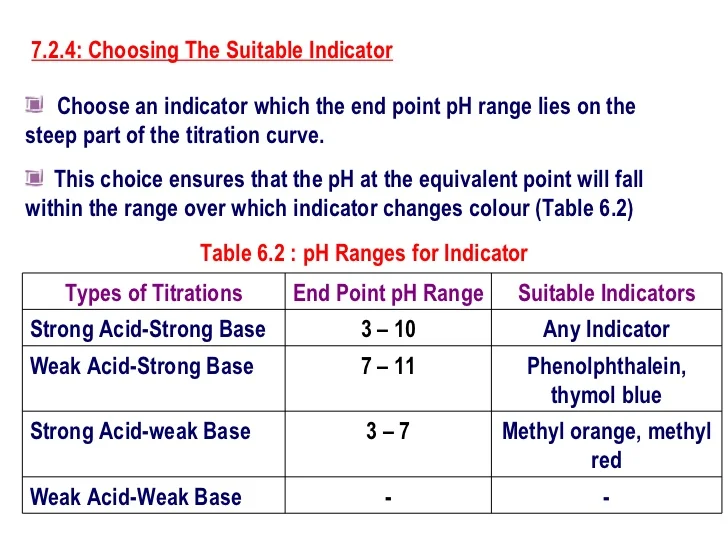
Titration Indicator End Point . The most obvious selection can be change in. All methods of the end point detection are based on the visible changes of the solution properties. The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. Otherwise, an indicator may be. Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: This page assumes that you know about ph curves for all the. End point detection in tiration. (4) the colour at the end point. If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed.
from www.slideshare.net
The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Otherwise, an indicator may be. (4) the colour at the end point. This page assumes that you know about ph curves for all the. If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed. The most obvious selection can be change in. All methods of the end point detection are based on the visible changes of the solution properties. End point detection in tiration.
Acid base titration
Titration Indicator End Point The most obvious selection can be change in. If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed. All methods of the end point detection are based on the visible changes of the solution properties. Using a ph probe and using a colour indicator. This page assumes that you know about ph curves for all the. (4) the colour at the end point. The most obvious selection can be change in. Otherwise, an indicator may be. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. End point detection in tiration.
From slideplayer.com
Titration Colour Changes ppt download Titration Indicator End Point The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. Otherwise, an indicator may be. Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: All methods of the end point. Titration Indicator End Point.
From mungfali.com
Acid Base Titration Indicator Titration Indicator End Point For a ph titration (between an acid and a base), there are two common ways to find the endpoint: If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed. The most obvious selection can be change in. Otherwise, an indicator may be. (4) the colour at the. Titration Indicator End Point.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Indicator End Point This page assumes that you know about ph curves for all the. Using a ph probe and using a colour indicator. If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed. The relatively broad range of phs over which an indicator changes color places additional limitations on. Titration Indicator End Point.
From www.youtube.com
Titration end point using phenolphthalein indicator YouTube Titration Indicator End Point All methods of the end point detection are based on the visible changes of the solution properties. Otherwise, an indicator may be. (4) the colour at the end point. The most obvious selection can be change in. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: This page assumes that. Titration Indicator End Point.
From www.numerade.com
SOLVED How can you improve your ability to see the indicator color Titration Indicator End Point Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed. All methods of the end point detection are based. Titration Indicator End Point.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Titration Indicator End Point This page assumes that you know about ph curves for all the. Using a ph probe and using a colour indicator. The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. For a ph titration (between an acid and a base), there are two common ways to find the. Titration Indicator End Point.
From www.slideshare.net
Acid base titration Titration Indicator End Point Otherwise, an indicator may be. The most obvious selection can be change in. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: This page assumes that you know about ph curves for all the. The relatively broad range of phs over which an indicator changes color places additional limitations on. Titration Indicator End Point.
From slideplayer.com
Acids and Bases. ppt download Titration Indicator End Point This page assumes that you know about ph curves for all the. The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the. Titration Indicator End Point.
From courses.lumenlearning.com
AcidBase Titrations Boundless Chemistry Titration Indicator End Point All methods of the end point detection are based on the visible changes of the solution properties. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The most obvious selection can be change in. End point detection in tiration. Otherwise, an indicator may be. If either the titrant or analyte. Titration Indicator End Point.
From www.animalia-life.club
Endpoint Titration Titration Indicator End Point If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed. This page assumes that you know about ph curves for all the. End point detection in tiration. All methods of the end point detection are based on the visible changes of the solution properties. The most obvious. Titration Indicator End Point.
From slideplayer.com
Unit 13 Acid, Bases, & Salts ppt download Titration Indicator End Point End point detection in tiration. (4) the colour at the end point. This page assumes that you know about ph curves for all the. Otherwise, an indicator may be. The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. All methods of the end point detection are based on. Titration Indicator End Point.
From philschatz.com
AcidBase Titrations · Chemistry Titration Indicator End Point End point detection in tiration. Using a ph probe and using a colour indicator. (4) the colour at the end point. The most obvious selection can be change in. The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. This page assumes that you know about ph curves for. Titration Indicator End Point.
From ar.inspiredpencil.com
Endpoint Titration Titration Indicator End Point If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed. The most obvious selection can be change in. Using a ph probe and using a colour indicator. The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal. Titration Indicator End Point.
From slideplayer.com
Titrations!. ppt download Titration Indicator End Point If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed. This page assumes that you know about ph curves for all the. End point detection in tiration. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The. Titration Indicator End Point.
From byjus.com
Study The pH Change In The Titration Of A Strong Base Using Universal Titration Indicator End Point This page assumes that you know about ph curves for all the. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed. Otherwise, an indicator may be. Using. Titration Indicator End Point.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Indicator End Point For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. This page assumes that you know about ph curves for all the. (4) the colour at the end point. Using. Titration Indicator End Point.
From in.pinterest.com
End Point Flashcard 10th Grade Science Self help book, Easy science Titration Indicator End Point The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed. (4) the colour at the end point. The most obvious selection can be change in. All. Titration Indicator End Point.
From www.researchgate.net
End point colours changes of titration with methanol extract and Titration Indicator End Point This page assumes that you know about ph curves for all the. End point detection in tiration. (4) the colour at the end point. All methods of the end point detection are based on the visible changes of the solution properties. For a ph titration (between an acid and a base), there are two common ways to find the endpoint:. Titration Indicator End Point.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Indicator End Point All methods of the end point detection are based on the visible changes of the solution properties. The most obvious selection can be change in. Using a ph probe and using a colour indicator. Otherwise, an indicator may be. End point detection in tiration. For a ph titration (between an acid and a base), there are two common ways to. Titration Indicator End Point.
From slideplayer.com
Chapter 19 Acids, Bases, and Salts ppt download Titration Indicator End Point Using a ph probe and using a colour indicator. The most obvious selection can be change in. (4) the colour at the end point. If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed. All methods of the end point detection are based on the visible changes. Titration Indicator End Point.
From mungfali.com
Acid Base Titration Indicator Titration Indicator End Point Otherwise, an indicator may be. Using a ph probe and using a colour indicator. End point detection in tiration. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. The. Titration Indicator End Point.
From www.slideserve.com
PPT CH 103 ACIDBASE TITRATIONS PowerPoint Presentation, free Titration Indicator End Point All methods of the end point detection are based on the visible changes of the solution properties. End point detection in tiration. The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. Otherwise, an indicator may be. (4) the colour at the end point. For a ph titration (between. Titration Indicator End Point.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Indicator End Point Otherwise, an indicator may be. The most obvious selection can be change in. End point detection in tiration. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. Using a. Titration Indicator End Point.
From www.alamy.com
Titration hires stock photography and images Alamy Titration Indicator End Point This page assumes that you know about ph curves for all the. The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed. End point detection in. Titration Indicator End Point.
From www.youtube.com
AcidBase Titration (Thymolphthalein end point) YouTube Titration Indicator End Point Otherwise, an indicator may be. (4) the colour at the end point. The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. End point detection in tiration. All methods of the end point detection are based on the visible changes of the solution properties. Using a ph probe and. Titration Indicator End Point.
From asdlib.org
Indicators for Complexation Titrations Image and Video Exchange Titration Indicator End Point Using a ph probe and using a colour indicator. The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. End point detection in tiration. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Otherwise, an indicator may be. The. Titration Indicator End Point.
From www.animalia-life.club
Endpoint Titration Titration Indicator End Point Otherwise, an indicator may be. The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. The most obvious selection can be. Titration Indicator End Point.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein Titration Indicator End Point This page assumes that you know about ph curves for all the. All methods of the end point detection are based on the visible changes of the solution properties. The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. End point detection in tiration. The most obvious selection can. Titration Indicator End Point.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2976284 Titration Indicator End Point Using a ph probe and using a colour indicator. The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. Otherwise, an indicator may be. The most obvious selection can be change in. (4) the colour at the end point. For a ph titration (between an acid and a base),. Titration Indicator End Point.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID9466916 Titration Indicator End Point All methods of the end point detection are based on the visible changes of the solution properties. The most obvious selection can be change in. Using a ph probe and using a colour indicator. If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed. This page assumes. Titration Indicator End Point.
From stock.adobe.com
Acidbase titration and phenolphthalein indicator Stock Vector Adobe Titration Indicator End Point (4) the colour at the end point. End point detection in tiration. The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed. The most obvious selection. Titration Indicator End Point.
From mirjamglessmer.com
Measuring the concentration of dissolved oxygen in sea water Part 3 Titration Indicator End Point The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. Otherwise, an indicator may be. Using a ph probe and using a colour indicator. This page assumes that you know about ph curves for all the. For a ph titration (between an acid and a base), there are two. Titration Indicator End Point.
From www.numerade.com
SOLVEDa. What is meant by the end point of a titration? b. What is the Titration Indicator End Point Using a ph probe and using a colour indicator. End point detection in tiration. All methods of the end point detection are based on the visible changes of the solution properties. If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed. (4) the colour at the end. Titration Indicator End Point.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Titration Indicator End Point All methods of the end point detection are based on the visible changes of the solution properties. End point detection in tiration. Otherwise, an indicator may be. This page assumes that you know about ph curves for all the. The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a.. Titration Indicator End Point.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Titration Indicator End Point End point detection in tiration. The relatively broad range of phs over which an indicator changes color places additional limitations on its ability to signal a. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: If either the titrant or analyte is colored, the equivalence point is evident from the. Titration Indicator End Point.