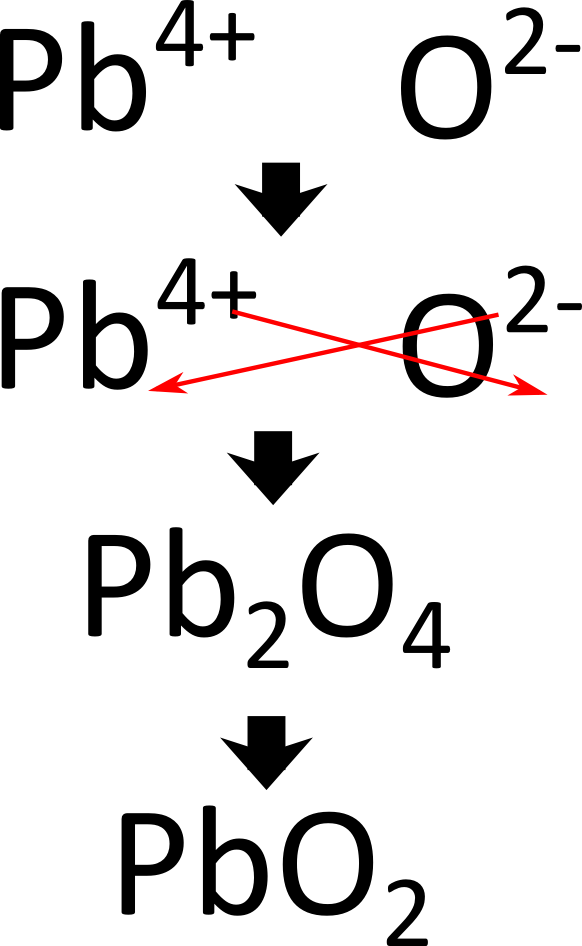
Lead (Iv) Chromate Formula . Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. the objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice. we use the principle of charge neutrality, that is, for an ionic compound to be stable its chemical formula must be neutral. Nist chemistry webbook the national institute of. — write the formula for lead (iv) oxide. Top data from nist standard reference database 69: By the end of this section, you will be able to: Define ionic and molecular (covalent) compounds. lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating.
from ck12.org
Top data from nist standard reference database 69: — write the formula for lead (iv) oxide. By the end of this section, you will be able to: Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Define ionic and molecular (covalent) compounds. the objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice. we use the principle of charge neutrality, that is, for an ionic compound to be stable its chemical formula must be neutral. Nist chemistry webbook the national institute of.
Ionic Compounds CK12 Foundation
Lead (Iv) Chromate Formula Define ionic and molecular (covalent) compounds. the objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice. lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. By the end of this section, you will be able to: Nist chemistry webbook the national institute of. — write the formula for lead (iv) oxide. Top data from nist standard reference database 69: we use the principle of charge neutrality, that is, for an ionic compound to be stable its chemical formula must be neutral. Define ionic and molecular (covalent) compounds. Exercise \ (\pageindex {2}\) write the chemical formula for an ionic.
From poliex.me
LEAD CHROMATE PbCrO4 Poliex Lead (Iv) Chromate Formula By the end of this section, you will be able to: — write the formula for lead (iv) oxide. Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. Top data from nist standard reference database 69: Define ionic and molecular (covalent) compounds. we use the principle of charge neutrality, that is, for an ionic compound to. Lead (Iv) Chromate Formula.
From www.fishersci.com
Lead(IV) fluoride, 99, Thermo Scientific Fisher Scientific Lead (Iv) Chromate Formula — write the formula for lead (iv) oxide. we use the principle of charge neutrality, that is, for an ionic compound to be stable its chemical formula must be neutral. Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. the objective of this and the next two sections is to teach you to write the. Lead (Iv) Chromate Formula.
From www.fishersci.ca
Lead(II) chromate, 98, Thermo Scientific Chemicals Fisher Scientific Lead (Iv) Chromate Formula By the end of this section, you will be able to: Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Nist chemistry webbook the national institute of. Define ionic and molecular (covalent) compounds. — write the formula. Lead (Iv) Chromate Formula.
From ck12.org
Ionic Compounds CK12 Foundation Lead (Iv) Chromate Formula — write the formula for lead (iv) oxide. Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. the objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice. lead chromate is a yellow, orange or red colored, crystalline, inorganic compound. Lead (Iv) Chromate Formula.
From www.numerade.com
SOLVED '3. iron (III) oxide 4 . carbon monoxide 5.calcium chloride 6 Lead (Iv) Chromate Formula the objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice. Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. — write the formula for lead (iv) oxide. Nist chemistry webbook the national institute of. Define ionic and molecular (covalent) compounds.. Lead (Iv) Chromate Formula.
From shayna-blogcoffey.blogspot.com
What Is the Formula for Tin Iv Chromate Lead (Iv) Chromate Formula Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. the objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice. By the end of this section, you will be able to: Define ionic and molecular (covalent) compounds. Top data from nist standard. Lead (Iv) Chromate Formula.
From www.slideshare.net
Lead chromate page 3 Lead (Iv) Chromate Formula Nist chemistry webbook the national institute of. — write the formula for lead (iv) oxide. Top data from nist standard reference database 69: lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. By the end of this section, you will be able to: Exercise \ (\pageindex {2}\) write. Lead (Iv) Chromate Formula.
From uspigment.com
Lead Chromate US Pigment Corporation Lead (Iv) Chromate Formula Nist chemistry webbook the national institute of. Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. Define ionic and molecular (covalent) compounds. — write the formula for lead (iv) oxide. lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. the objective of this and the. Lead (Iv) Chromate Formula.
From dir.indiamart.com
Lead Chromate at Best Price in India Lead (Iv) Chromate Formula By the end of this section, you will be able to: lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Define ionic and molecular (covalent) compounds. the objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound. Lead (Iv) Chromate Formula.
From testbook.com
Lead (IV) Acetate Formula, Properties, Structure, and Uses Lead (Iv) Chromate Formula By the end of this section, you will be able to: the objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice. Define ionic and molecular (covalent) compounds. Nist chemistry webbook the national institute of. lead chromate is a yellow, orange or red. Lead (Iv) Chromate Formula.
From ar.inspiredpencil.com
Chromate Lewis Structure Lead (Iv) Chromate Formula the objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice. Top data from nist standard reference database 69: Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. Define ionic and molecular (covalent) compounds. we use the principle of charge neutrality,. Lead (Iv) Chromate Formula.
From www.slideshare.net
Lead chromate page 6 Lead (Iv) Chromate Formula Nist chemistry webbook the national institute of. Define ionic and molecular (covalent) compounds. — write the formula for lead (iv) oxide. we use the principle of charge neutrality, that is, for an ionic compound to be stable its chemical formula must be neutral. lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits. Lead (Iv) Chromate Formula.
From www.sciencephoto.com
Lead chromate and potassium dichromate Stock Image A500/0090 Lead (Iv) Chromate Formula lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Nist chemistry webbook the national institute of. Top data from nist standard reference database 69: the objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its. Lead (Iv) Chromate Formula.
From slideplayer.com
Semester I Review. ppt download Lead (Iv) Chromate Formula Define ionic and molecular (covalent) compounds. we use the principle of charge neutrality, that is, for an ionic compound to be stable its chemical formula must be neutral. the objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice. Exercise \ (\pageindex {2}\). Lead (Iv) Chromate Formula.
From www.labdepotinc.com
Lead Chromate, Powder, Reagent, ACS Lead (Iv) Chromate Formula Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. Nist chemistry webbook the national institute of. the objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice. we use the principle of charge neutrality, that is, for an ionic compound to. Lead (Iv) Chromate Formula.
From www.t3db.ca
T3DB Lead molybdenum chromate Lead (Iv) Chromate Formula Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Nist chemistry webbook the national institute of. — write the formula for lead (iv) oxide. By the end of this section, you will be able to: we. Lead (Iv) Chromate Formula.
From www.dreamstime.com
Skeletal Formula of Chromate Anion, Chemical Structure. Stock Vector Lead (Iv) Chromate Formula we use the principle of charge neutrality, that is, for an ionic compound to be stable its chemical formula must be neutral. Nist chemistry webbook the national institute of. the objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice. Top data from. Lead (Iv) Chromate Formula.
From commons.wikimedia.org
CategoryLead chromate Wikimedia Commons Lead (Iv) Chromate Formula Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. the objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice. — write the formula for lead (iv) oxide. Nist chemistry webbook the national institute of. lead chromate is a yellow,. Lead (Iv) Chromate Formula.
From slideplayer.com
Atoms, Molecules, and Ions ppt download Lead (Iv) Chromate Formula lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. we use the principle of charge neutrality, that is, for an ionic compound to be stable its chemical formula must be neutral. Define ionic and molecular (covalent) compounds. the objective of this and the next two sections is. Lead (Iv) Chromate Formula.
From www.researchgate.net
Lead chromate induced cytotoxicity in the 24h treated AA8, EM9, and Lead (Iv) Chromate Formula By the end of this section, you will be able to: we use the principle of charge neutrality, that is, for an ionic compound to be stable its chemical formula must be neutral. — write the formula for lead (iv) oxide. the objective of this and the next two sections is to teach you to write the. Lead (Iv) Chromate Formula.
From www.youtube.com
How to write the formula for lead (IV) iodide YouTube Lead (Iv) Chromate Formula Define ionic and molecular (covalent) compounds. lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Nist chemistry webbook the national institute of. — write the formula for lead (iv) oxide. the objective of this and the next two sections is to teach you to write the formula. Lead (Iv) Chromate Formula.
From www.youtube.com
How to Write the Formula for Lead (IV) chromate YouTube Lead (Iv) Chromate Formula Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. — write the formula for lead (iv) oxide. By the end of this section, you will be able to: Top data from nist standard reference database 69: lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Define. Lead (Iv) Chromate Formula.
From www.slideshare.net
Lead chromate page 2 Lead (Iv) Chromate Formula lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Define ionic and molecular (covalent) compounds. Top data from nist standard reference database 69: Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. — write the formula for lead (iv) oxide. we use the principle of. Lead (Iv) Chromate Formula.
From www.youtube.com
Lead IV SulfateHow to write chemical formula of Lead IV SulfateLead Lead (Iv) Chromate Formula — write the formula for lead (iv) oxide. Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. By the end of this section, you will be able to: we use the principle of charge neutrality, that is, for an ionic compound to be stable its chemical formula must be neutral. the objective of this and. Lead (Iv) Chromate Formula.
From sielc.com
Lead(IV) acetate SIELC Lead (Iv) Chromate Formula Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. Nist chemistry webbook the national institute of. we use the principle of charge neutrality, that is, for an ionic compound to be stable its chemical formula must be neutral. Top data from nist standard reference database 69: the objective of this and the next two sections is. Lead (Iv) Chromate Formula.
From www.alamy.com
PbCrO4 lead chromate CAS 7758976 chemical substance in white plastic Lead (Iv) Chromate Formula Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. By the end of this section, you will be able to: — write the formula for lead (iv) oxide. we use the principle of charge neutrality, that is, for an ionic compound to be stable its chemical formula must be neutral. the objective of this and. Lead (Iv) Chromate Formula.
From www.fishersci.com
Lead Chromate, Powder, ACS, 98, Spectrum Chemical, Quantity 125 g Lead (Iv) Chromate Formula lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. — write the formula for lead (iv) oxide. By the end of this section, you will be able to: Nist chemistry webbook the national institute of. Top data from nist standard reference database 69: the objective of this. Lead (Iv) Chromate Formula.
From www.alamy.com
Lead (II,IV) oxide, Potassium Chromate and Organic Curcuma Powder in Lead (Iv) Chromate Formula — write the formula for lead (iv) oxide. By the end of this section, you will be able to: Define ionic and molecular (covalent) compounds. lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Top data from nist standard reference database 69: Exercise \ (\pageindex {2}\) write the. Lead (Iv) Chromate Formula.
From www.labdepotinc.com
Lead Chromate Lead (Iv) Chromate Formula we use the principle of charge neutrality, that is, for an ionic compound to be stable its chemical formula must be neutral. Top data from nist standard reference database 69: By the end of this section, you will be able to: Define ionic and molecular (covalent) compounds. the objective of this and the next two sections is to. Lead (Iv) Chromate Formula.
From antwanmeowcosta.blogspot.com
What Is the Formula for Lead Iv Sulfite Lead (Iv) Chromate Formula the objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice. lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. By the end of this section, you will be able to: Top data. Lead (Iv) Chromate Formula.
From slideplayer.com
Chemical Formulae symbols, naming, writing formulae. ppt download Lead (Iv) Chromate Formula Nist chemistry webbook the national institute of. By the end of this section, you will be able to: Top data from nist standard reference database 69: Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. — write the formula for lead (iv) oxide. we use the principle of charge neutrality, that is, for an ionic compound. Lead (Iv) Chromate Formula.
From www.mingyuanchemical.com
Lead chromate 7758976 Lead (Iv) Chromate Formula the objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice. Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Nist chemistry. Lead (Iv) Chromate Formula.
From www.fishersci.fi
Lead(IV) oxide, Brown to black powder, 97.0 min., Alfa Aesar™ Other Lead (Iv) Chromate Formula Top data from nist standard reference database 69: lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. Nist chemistry webbook the national institute of. By the end of this section, you will be able to: we use. Lead (Iv) Chromate Formula.
From www.pw.live
Lead IV Oxide Formula, Structure, Properties, Uses Lead (Iv) Chromate Formula Top data from nist standard reference database 69: lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. By the end of this section, you will be able to: — write the formula for lead (iv) oxide. Define ionic and molecular (covalent) compounds. Nist chemistry webbook the national institute. Lead (Iv) Chromate Formula.
From www.youtube.com
How to Write the Formula for Lead (IV) carbonate YouTube Lead (Iv) Chromate Formula Top data from nist standard reference database 69: Define ionic and molecular (covalent) compounds. Nist chemistry webbook the national institute of. — write the formula for lead (iv) oxide. Exercise \ (\pageindex {2}\) write the chemical formula for an ionic. we use the principle of charge neutrality, that is, for an ionic compound to be stable its chemical. Lead (Iv) Chromate Formula.