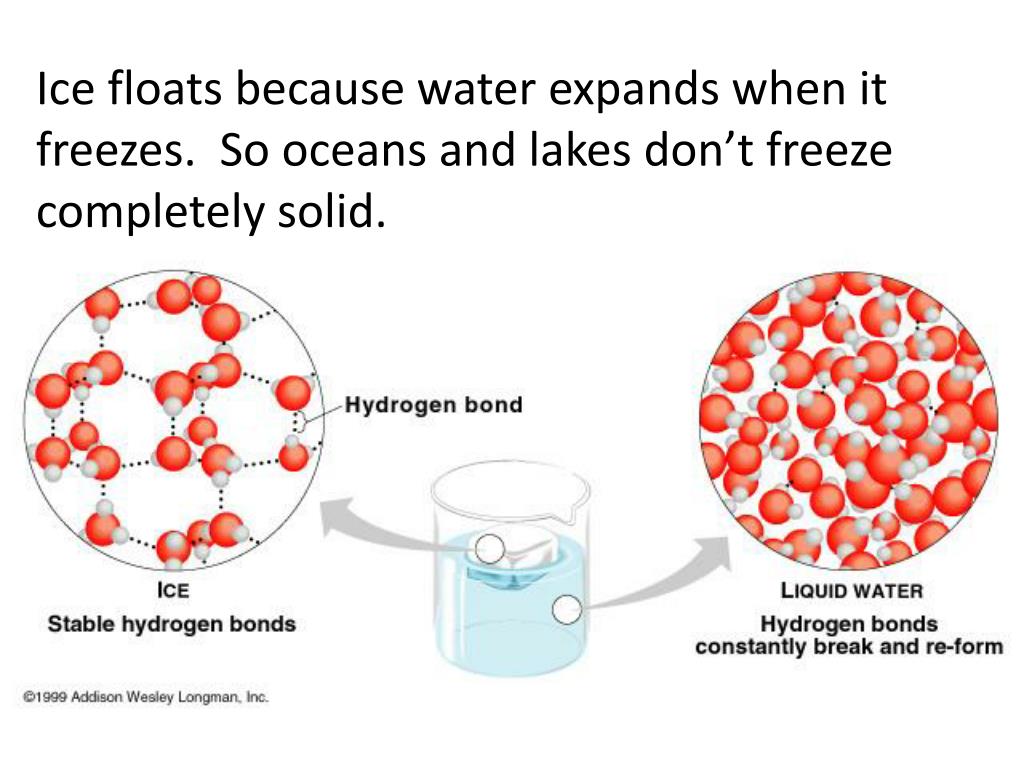
Why Does Ice Float On Water Density . view full lesson: discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. but as it gets colder than 4 o c, water starts to expand causing a decrease in density. at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. On further cooling, it turns into ice at 0 o c, becoming 9%. ice floats because it is about 9% less dense than liquid water. the scientific explanation for why ice floats on water is based on the concept of density. Water, however, reaches its maximum. In other words, ice takes up about 9% more space.
from dxofacpez.blob.core.windows.net
at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. the scientific explanation for why ice floats on water is based on the concept of density. Water, however, reaches its maximum. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. view full lesson: but as it gets colder than 4 o c, water starts to expand causing a decrease in density. discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. ice floats because it is about 9% less dense than liquid water. On further cooling, it turns into ice at 0 o c, becoming 9%.
Why Do Ice Cubes Float In Water at William Schmid blog
Why Does Ice Float On Water Density On further cooling, it turns into ice at 0 o c, becoming 9%. the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. but as it gets colder than 4 o c, water starts to expand causing a decrease in density. In other words, ice takes up about 9% more space. view full lesson: at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. On further cooling, it turns into ice at 0 o c, becoming 9%. Water, however, reaches its maximum. discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. the scientific explanation for why ice floats on water is based on the concept of density. ice floats because it is about 9% less dense than liquid water.
From www.slideserve.com
PPT Chapter 17 Water and Aqueous Systems PowerPoint Presentation Why Does Ice Float On Water Density On further cooling, it turns into ice at 0 o c, becoming 9%. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. but as it gets colder than 4. Why Does Ice Float On Water Density.
From www.slideserve.com
PPT Density, Mass, & Volume PowerPoint Presentation, free download Why Does Ice Float On Water Density In other words, ice takes up about 9% more space. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. On further cooling, it turns into ice at 0 o c, becoming 9%. the scientific explanation for why ice floats on water is based on the concept of density. discover. Why Does Ice Float On Water Density.
From www.chegg.com
Solved 5. Why does ice float on water? 1 6. What factors Why Does Ice Float On Water Density On further cooling, it turns into ice at 0 o c, becoming 9%. but as it gets colder than 4 o c, water starts to expand causing a decrease in density. ice floats because it is about 9% less dense than liquid water. discover the unusual properties of water that make ice less dense and how this. Why Does Ice Float On Water Density.
From www.youtube.com
what is Density Why does ice float on water? SI unit, Formula Why Does Ice Float On Water Density In other words, ice takes up about 9% more space. ice floats because it is about 9% less dense than liquid water. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. but as it gets colder than 4 o c, water starts to expand causing a decrease in density.. Why Does Ice Float On Water Density.
From www.scienceabc.com
Why Does Ice Float On Water? » ScienceABC Why Does Ice Float On Water Density the scientific explanation for why ice floats on water is based on the concept of density. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. but as it gets colder than 4 o c, water starts to expand causing a decrease in density. discover the unusual properties of. Why Does Ice Float On Water Density.
From www.slideserve.com
PPT Aim How do we calculate density? PowerPoint Presentation, free Why Does Ice Float On Water Density discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. but as it gets colder than 4 o c, water starts to expand causing a decrease in density. ice floats because it is about 9% less dense than liquid water. On further cooling, it turns into ice. Why Does Ice Float On Water Density.
From www.youtube.com
Why ice floats on liquid water? Why volume of water increases on Why Does Ice Float On Water Density Water, however, reaches its maximum. the scientific explanation for why ice floats on water is based on the concept of density. view full lesson: the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. at zero degrees, i.e., the temperature at which water turns into ice, the. Why Does Ice Float On Water Density.
From dxoxkxrns.blob.core.windows.net
Why Is It Important To Ecosystems That Ice Floats In Liquid Water at Why Does Ice Float On Water Density ice floats because it is about 9% less dense than liquid water. In other words, ice takes up about 9% more space. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. but as it gets colder than 4 o c, water starts to expand causing a decrease in density.. Why Does Ice Float On Water Density.
From www.slideserve.com
PPT Show the Arrangement of Particles in Solid, Liquid and Gaseous Why Does Ice Float On Water Density In other words, ice takes up about 9% more space. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. On further cooling, it turns into ice at 0 o c, becoming. Why Does Ice Float On Water Density.
From www.slideserve.com
PPT Chapter 3 Chemical and Physical Features of Seawater and the Why Does Ice Float On Water Density view full lesson: In other words, ice takes up about 9% more space. On further cooling, it turns into ice at 0 o c, becoming 9%. the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. Water, however, reaches its maximum. at zero degrees, i.e., the temperature at. Why Does Ice Float On Water Density.
From www.slideserve.com
PPT C H A P T E R 11 Fluids PowerPoint Presentation, free download Why Does Ice Float On Water Density discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. the scientific explanation for why ice floats on water is based on the concept of density. at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. . Why Does Ice Float On Water Density.
From www.youtube.com
why does ice float on water? water has max density at 4degree c Why Does Ice Float On Water Density Water, however, reaches its maximum. at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. ice floats because it is about 9% less dense than liquid water. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. view full. Why Does Ice Float On Water Density.
From techiescientist.com
Why Does Ice Float on Water? Techiescientist Why Does Ice Float On Water Density discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. the scientific explanation for why ice floats on water is based on the concept of density. but as it gets colder than 4 o c, water starts to expand causing a decrease in density. the fact. Why Does Ice Float On Water Density.
From www.wonderopolis.org
Why Does Ice Float in Water? Wonderopolis Why Does Ice Float On Water Density as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change.. Why Does Ice Float On Water Density.
From www.youtube.com
Why Ice Floats on Water YouTube Why Does Ice Float On Water Density discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. In other words, ice takes up about 9% more space. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. but as it gets colder than 4 o c, water. Why Does Ice Float On Water Density.
From www.youtube.com
Why does ice float on water? Detailed Explanation YouTube Why Does Ice Float On Water Density the scientific explanation for why ice floats on water is based on the concept of density. the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. In other words, ice takes up about 9% more space. discover the unusual properties of water that make ice less dense and. Why Does Ice Float On Water Density.
From www.livescience.com
Why does ice float? Live Science Why Does Ice Float On Water Density the scientific explanation for why ice floats on water is based on the concept of density. In other words, ice takes up about 9% more space. at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. Water, however, reaches its maximum. On further cooling, it turns into ice. Why Does Ice Float On Water Density.
From www.thoughtco.com
Why Does Ice Float? Ice and the Density of Water Why Does Ice Float On Water Density the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. view full lesson: Water, however, reaches its maximum. but as it gets colder than 4 o c, water starts to expand causing a decrease in density. at zero degrees, i.e., the temperature at which water turns into. Why Does Ice Float On Water Density.
From slidesharenow.blogspot.com
Why Does Ice Float On Water slideshare Why Does Ice Float On Water Density the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. On further cooling, it turns into ice at 0 o c, becoming 9%. view full lesson: but as it gets colder than 4 o c, water starts to expand causing a decrease in density. as it freezes,. Why Does Ice Float On Water Density.
From www.slideshare.net
Why does ice float on water? Why Does Ice Float On Water Density the scientific explanation for why ice floats on water is based on the concept of density. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. On further cooling, it turns into ice at 0 o c, becoming 9%. Water, however, reaches its maximum. the fact that ice floats in. Why Does Ice Float On Water Density.
From www.slideserve.com
PPT L14 Fluids [3] PowerPoint Presentation, free download ID5631091 Why Does Ice Float On Water Density but as it gets colder than 4 o c, water starts to expand causing a decrease in density. ice floats because it is about 9% less dense than liquid water. view full lesson: the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. Water, however, reaches its. Why Does Ice Float On Water Density.
From www.scienceabc.com
Why Does Ice Float On Water? » ScienceABC Why Does Ice Float On Water Density On further cooling, it turns into ice at 0 o c, becoming 9%. view full lesson: the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. In other words, ice takes up about 9% more space. the scientific explanation for why ice floats on water is based on. Why Does Ice Float On Water Density.
From dxofacpez.blob.core.windows.net
Why Do Ice Cubes Float In Water at William Schmid blog Why Does Ice Float On Water Density but as it gets colder than 4 o c, water starts to expand causing a decrease in density. ice floats because it is about 9% less dense than liquid water. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. Water, however, reaches its maximum. discover the unusual properties. Why Does Ice Float On Water Density.
From slidesharenow.blogspot.com
Why Does Ice Float On Water slideshare Why Does Ice Float On Water Density but as it gets colder than 4 o c, water starts to expand causing a decrease in density. the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. view full lesson: In other words, ice takes up about 9% more space. at zero degrees, i.e., the temperature. Why Does Ice Float On Water Density.
From www.dreamstime.com
Why Does Ice Float on Water Infographic Diagram Stock Vector Why Does Ice Float On Water Density the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. but as it gets colder than 4 o c, water starts to expand causing a decrease in density. Water, however, reaches its maximum. at zero degrees, i.e., the temperature at which water turns into ice, the density of. Why Does Ice Float On Water Density.
From www.youtube.com
Why does ice float in water? Zaidan and Charles Morton YouTube Why Does Ice Float On Water Density view full lesson: On further cooling, it turns into ice at 0 o c, becoming 9%. the scientific explanation for why ice floats on water is based on the concept of density. discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. at zero degrees, i.e.,. Why Does Ice Float On Water Density.
From techiescientist.com
Why Does Ice Float on Water? Techiescientist Why Does Ice Float On Water Density On further cooling, it turns into ice at 0 o c, becoming 9%. but as it gets colder than 4 o c, water starts to expand causing a decrease in density. the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. view full lesson: as it freezes,. Why Does Ice Float On Water Density.
From memphisice.com
Why Does Ice Float on Water? Memphis Ice Why Does Ice Float On Water Density In other words, ice takes up about 9% more space. Water, however, reaches its maximum. discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. but as it gets colder than 4 o c, water starts to expand causing a decrease in density. the scientific explanation for. Why Does Ice Float On Water Density.
From www.slideserve.com
PPT Chapter 11 Fluid Statics PowerPoint Presentation, free download Why Does Ice Float On Water Density ice floats because it is about 9% less dense than liquid water. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. view full lesson: discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. In other words, ice. Why Does Ice Float On Water Density.
From academichelp.net
Why Does Ice Float on Water? The Science Behind It Explained Why Does Ice Float On Water Density the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. ice floats because it is about 9% less dense than liquid water. view full lesson: Water, however, reaches its maximum. the scientific explanation for why ice floats on water is based on the concept of density. . Why Does Ice Float On Water Density.
From www.youtube.com
Density Why Ice floats on Water? YouTube Why Does Ice Float On Water Density On further cooling, it turns into ice at 0 o c, becoming 9%. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. ice floats because it is about 9% less dense than liquid water. but as it gets colder than 4 o c, water starts to expand causing a. Why Does Ice Float On Water Density.
From www.slideserve.com
PPT L14 Fluids [3] PowerPoint Presentation, free download ID2718491 Why Does Ice Float On Water Density Water, however, reaches its maximum. view full lesson: at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. the scientific explanation for why ice floats on water. Why Does Ice Float On Water Density.
From www.worldatlas.com
Why Does Ice Float? WorldAtlas Why Does Ice Float On Water Density the scientific explanation for why ice floats on water is based on the concept of density. at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. view full lesson: as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense.. Why Does Ice Float On Water Density.
From www.slideserve.com
PPT Chemical and Physical Features of Seawater and the World Ocean Why Does Ice Float On Water Density discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. Water, however, reaches its maximum. ice floats because it is about 9% less dense than liquid water. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. view full. Why Does Ice Float On Water Density.
From dxozadxer.blob.core.windows.net
Does Ice Float On Water Because Of Cohesion at Thanh Jones blog Why Does Ice Float On Water Density On further cooling, it turns into ice at 0 o c, becoming 9%. the fact that ice floats in water is a bit strange, because most substances are denser when they're solids. as it freezes, sea ice exudes salt and makes the water beneath it extremely salty and dense. the scientific explanation for why ice floats on. Why Does Ice Float On Water Density.