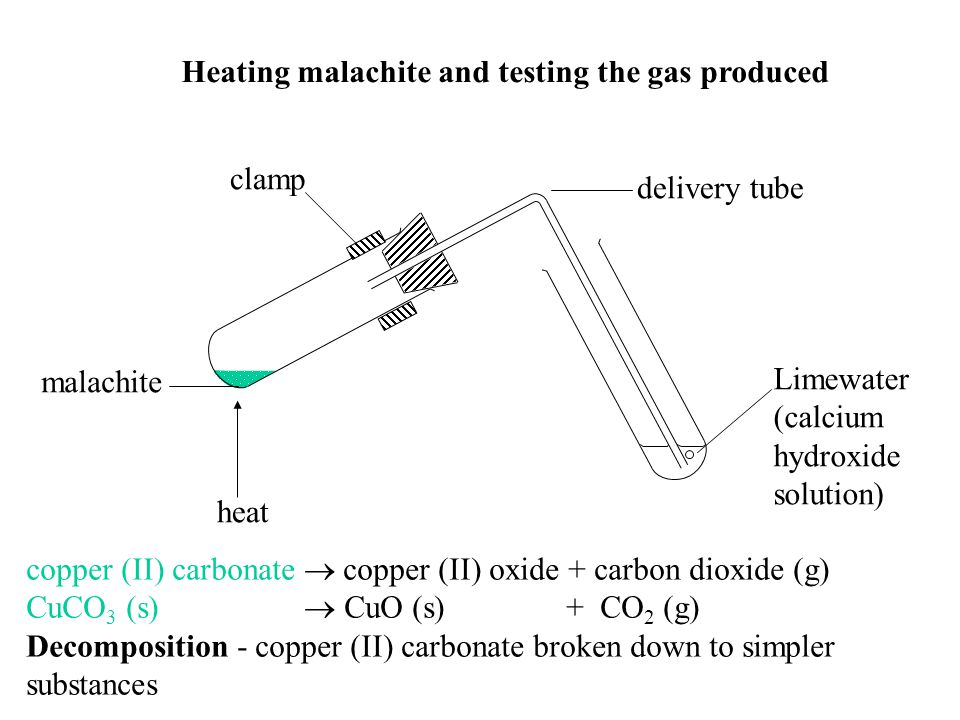
Lead Carbonate Thermal Decomposition Word Equation . Lead carbonate → lead oxide + carbon dioxide. Copper carbonate produces copper oxide, plus carbon. what is the difference between thermal dissociation and thermal decomposition? State the type of reactions for the. We’ll explain why thermal decomposition is. the word equation for this reaction is written like this: Revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Metal carbonates decompose when heated. The aim of this experiment is to compare the reactivity of some different metal. Some carbonates are more reactive than others. consider the following word equation for a thermal decomposition reaction: in this video, we will learn how to describe and give examples of thermal decomposition reactions. consider the following word equation for a thermal decomposition reaction:
from www.online-sciences.com
the word equation for this reaction is written like this: compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. We’ll explain why thermal decomposition is. Copper carbonate produces copper oxide, plus carbon. Revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry. State the type of reactions for the. Lead carbonate → lead oxide + carbon dioxide. in this video, we will learn how to describe and give examples of thermal decomposition reactions. consider the following word equation for a thermal decomposition reaction: Some carbonates are more reactive than others.
Types of chemical reactions and Thermal reactions
Lead Carbonate Thermal Decomposition Word Equation in this video, we will learn how to describe and give examples of thermal decomposition reactions. consider the following word equation for a thermal decomposition reaction: We’ll explain why thermal decomposition is. Lead carbonate → lead oxide + carbon dioxide. Revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry. State the type of reactions for the. in this video, we will learn how to describe and give examples of thermal decomposition reactions. what is the difference between thermal dissociation and thermal decomposition? Metal carbonates decompose when heated. Some carbonates are more reactive than others. Copper carbonate produces copper oxide, plus carbon. The aim of this experiment is to compare the reactivity of some different metal. consider the following word equation for a thermal decomposition reaction: compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. the word equation for this reaction is written like this:
From www.youtube.com
How to Write the Formula for Lead (II) carbonate YouTube Lead Carbonate Thermal Decomposition Word Equation Metal carbonates decompose when heated. in this video, we will learn how to describe and give examples of thermal decomposition reactions. consider the following word equation for a thermal decomposition reaction: compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. consider. Lead Carbonate Thermal Decomposition Word Equation.
From brainly.in
Write the balanced chemical equation of calcium carbonate on Lead Carbonate Thermal Decomposition Word Equation Lead carbonate → lead oxide + carbon dioxide. State the type of reactions for the. in this video, we will learn how to describe and give examples of thermal decomposition reactions. We’ll explain why thermal decomposition is. the word equation for this reaction is written like this: Copper carbonate produces copper oxide, plus carbon. The aim of this. Lead Carbonate Thermal Decomposition Word Equation.
From www.youtube.com
How to write the equation for PbCO3 + H2O Lead (II) carbonate + Water Lead Carbonate Thermal Decomposition Word Equation the word equation for this reaction is written like this: Lead carbonate → lead oxide + carbon dioxide. consider the following word equation for a thermal decomposition reaction: Copper carbonate produces copper oxide, plus carbon. what is the difference between thermal dissociation and thermal decomposition? compare the thermal stabilities of carbonates of reactive metals, like sodium. Lead Carbonate Thermal Decomposition Word Equation.
From www.slideserve.com
PPT CALCIUM CARBONATE A guide for GCSE students PowerPoint Lead Carbonate Thermal Decomposition Word Equation Some carbonates are more reactive than others. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. The aim of this experiment is to compare the reactivity of some different metal. State the type of reactions for the. Lead carbonate → lead oxide + carbon. Lead Carbonate Thermal Decomposition Word Equation.
From www.youtube.com
of Sodium carbonate (balancing equation) YouTube Lead Carbonate Thermal Decomposition Word Equation Some carbonates are more reactive than others. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. the word equation for this reaction is written like this: State the type of reactions for the. consider the following word equation for a thermal decomposition. Lead Carbonate Thermal Decomposition Word Equation.
From www.numerade.com
SOLVED with the help of chemical equation, show thermal Lead Carbonate Thermal Decomposition Word Equation compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. in this video, we will learn how to describe and give examples of thermal decomposition reactions. Some carbonates are more reactive than others. We’ll explain why thermal decomposition is. the word equation for. Lead Carbonate Thermal Decomposition Word Equation.
From studylib.net
Thermal worksheet (lower) Lead Carbonate Thermal Decomposition Word Equation consider the following word equation for a thermal decomposition reaction: Copper carbonate produces copper oxide, plus carbon. consider the following word equation for a thermal decomposition reaction: Lead carbonate → lead oxide + carbon dioxide. We’ll explain why thermal decomposition is. in this video, we will learn how to describe and give examples of thermal decomposition reactions.. Lead Carbonate Thermal Decomposition Word Equation.
From www.youtube.com
THERMAL REACTIONS/HEATING EFFECT ON AMMONIUM SALTS/TRICKS Lead Carbonate Thermal Decomposition Word Equation The aim of this experiment is to compare the reactivity of some different metal. what is the difference between thermal dissociation and thermal decomposition? State the type of reactions for the. Metal carbonates decompose when heated. consider the following word equation for a thermal decomposition reaction: Copper carbonate produces copper oxide, plus carbon. consider the following word. Lead Carbonate Thermal Decomposition Word Equation.
From www.youtube.com
Heating Lead carbonate YouTube Lead Carbonate Thermal Decomposition Word Equation what is the difference between thermal dissociation and thermal decomposition? consider the following word equation for a thermal decomposition reaction: Lead carbonate → lead oxide + carbon dioxide. Metal carbonates decompose when heated. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper.. Lead Carbonate Thermal Decomposition Word Equation.
From www.markedbyteachers.com
Thermal of Copper Carbonate ALevel Science Marked by Lead Carbonate Thermal Decomposition Word Equation what is the difference between thermal dissociation and thermal decomposition? consider the following word equation for a thermal decomposition reaction: We’ll explain why thermal decomposition is. consider the following word equation for a thermal decomposition reaction: Some carbonates are more reactive than others. Metal carbonates decompose when heated. compare the thermal stabilities of carbonates of reactive. Lead Carbonate Thermal Decomposition Word Equation.
From www.chegg.com
Solved (i) The reaction below show the of lead Lead Carbonate Thermal Decomposition Word Equation Revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry. Some carbonates are more reactive than others. the word equation for this reaction is written like this: consider the following word equation for a thermal decomposition reaction: Lead carbonate → lead oxide + carbon dioxide. compare the thermal stabilities of carbonates. Lead Carbonate Thermal Decomposition Word Equation.
From www.numerade.com
SOLVEDWrite balanced equations for the thermal of Lead Carbonate Thermal Decomposition Word Equation in this video, we will learn how to describe and give examples of thermal decomposition reactions. Copper carbonate produces copper oxide, plus carbon. Lead carbonate → lead oxide + carbon dioxide. the word equation for this reaction is written like this: The aim of this experiment is to compare the reactivity of some different metal. Some carbonates are. Lead Carbonate Thermal Decomposition Word Equation.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID6307470 Lead Carbonate Thermal Decomposition Word Equation Revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry. the word equation for this reaction is written like this: what is the difference between thermal dissociation and thermal decomposition? in this video, we will learn how to describe and give examples of thermal decomposition reactions. Some carbonates are more reactive. Lead Carbonate Thermal Decomposition Word Equation.
From exobokkbu.blob.core.windows.net
Thermal Of Lead(Ii) Nitrate at Cheryl Lopez blog Lead Carbonate Thermal Decomposition Word Equation Copper carbonate produces copper oxide, plus carbon. State the type of reactions for the. in this video, we will learn how to describe and give examples of thermal decomposition reactions. Some carbonates are more reactive than others. Lead carbonate → lead oxide + carbon dioxide. The aim of this experiment is to compare the reactivity of some different metal.. Lead Carbonate Thermal Decomposition Word Equation.
From edu.rsc.org
Thermal of metal carbonates Experiment RSC Education Lead Carbonate Thermal Decomposition Word Equation The aim of this experiment is to compare the reactivity of some different metal. consider the following word equation for a thermal decomposition reaction: the word equation for this reaction is written like this: in this video, we will learn how to describe and give examples of thermal decomposition reactions. We’ll explain why thermal decomposition is. . Lead Carbonate Thermal Decomposition Word Equation.
From www.online-sciences.com
Types of chemical reactions and Thermal reactions Lead Carbonate Thermal Decomposition Word Equation the word equation for this reaction is written like this: consider the following word equation for a thermal decomposition reaction: We’ll explain why thermal decomposition is. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. The aim of this experiment is to. Lead Carbonate Thermal Decomposition Word Equation.
From www.youtube.com
Thermal of Carbonates YouTube Lead Carbonate Thermal Decomposition Word Equation consider the following word equation for a thermal decomposition reaction: what is the difference between thermal dissociation and thermal decomposition? The aim of this experiment is to compare the reactivity of some different metal. Metal carbonates decompose when heated. We’ll explain why thermal decomposition is. Revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie. Lead Carbonate Thermal Decomposition Word Equation.
From www.slideserve.com
PPT Thermal PowerPoint Presentation ID544610 Lead Carbonate Thermal Decomposition Word Equation compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. State the type of reactions for the. consider the following word equation for a thermal decomposition reaction: Revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry. Some. Lead Carbonate Thermal Decomposition Word Equation.
From www.markedbyteachers.com
Determine which of two equations below is the correct equation of the Lead Carbonate Thermal Decomposition Word Equation The aim of this experiment is to compare the reactivity of some different metal. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. We’ll explain why thermal decomposition is. State the type of reactions for the. the word equation for this reaction is. Lead Carbonate Thermal Decomposition Word Equation.
From www.numerade.com
Lead (II) carbonate to form lead (II) oxide and carbon Lead Carbonate Thermal Decomposition Word Equation Metal carbonates decompose when heated. Revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry. Copper carbonate produces copper oxide, plus carbon. We’ll explain why thermal decomposition is. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. . Lead Carbonate Thermal Decomposition Word Equation.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free Lead Carbonate Thermal Decomposition Word Equation in this video, we will learn how to describe and give examples of thermal decomposition reactions. The aim of this experiment is to compare the reactivity of some different metal. consider the following word equation for a thermal decomposition reaction: Copper carbonate produces copper oxide, plus carbon. consider the following word equation for a thermal decomposition reaction:. Lead Carbonate Thermal Decomposition Word Equation.
From www.slideserve.com
PPT What is a chemical reaction? PowerPoint Presentation, free Lead Carbonate Thermal Decomposition Word Equation what is the difference between thermal dissociation and thermal decomposition? consider the following word equation for a thermal decomposition reaction: Lead carbonate → lead oxide + carbon dioxide. in this video, we will learn how to describe and give examples of thermal decomposition reactions. Some carbonates are more reactive than others. The aim of this experiment is. Lead Carbonate Thermal Decomposition Word Equation.
From www.slideserve.com
PPT CHEMICALS IN ACTION PowerPoint Presentation, free download ID Lead Carbonate Thermal Decomposition Word Equation We’ll explain why thermal decomposition is. the word equation for this reaction is written like this: Copper carbonate produces copper oxide, plus carbon. Revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry. consider the following word equation for a thermal decomposition reaction: in this video, we will learn how to. Lead Carbonate Thermal Decomposition Word Equation.
From www.slideserve.com
PPT Thermal of carbonates PowerPoint Presentation, free Lead Carbonate Thermal Decomposition Word Equation the word equation for this reaction is written like this: Metal carbonates decompose when heated. Copper carbonate produces copper oxide, plus carbon. consider the following word equation for a thermal decomposition reaction: compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. We’ll. Lead Carbonate Thermal Decomposition Word Equation.
From www.chegg.com
Solved Carbonate Thermal (two parts) Determine Lead Carbonate Thermal Decomposition Word Equation Revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry. State the type of reactions for the. We’ll explain why thermal decomposition is. Lead carbonate → lead oxide + carbon dioxide. The aim of this experiment is to compare the reactivity of some different metal. in this video, we will learn how to. Lead Carbonate Thermal Decomposition Word Equation.
From www.slideserve.com
PPT Limestone & its uses PowerPoint Presentation, free download ID Lead Carbonate Thermal Decomposition Word Equation what is the difference between thermal dissociation and thermal decomposition? The aim of this experiment is to compare the reactivity of some different metal. Revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry. Copper carbonate produces copper oxide, plus carbon. in this video, we will learn how to describe and give. Lead Carbonate Thermal Decomposition Word Equation.
From www.slideserve.com
PPT Thermal of carbonates PowerPoint Presentation, free Lead Carbonate Thermal Decomposition Word Equation Some carbonates are more reactive than others. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Copper carbonate produces copper oxide, plus carbon. The aim of this experiment is to compare the reactivity of some different metal. Revision notes on 2.2.3 thermal decomposition of. Lead Carbonate Thermal Decomposition Word Equation.
From studylib.net
File Lead Carbonate Thermal Decomposition Word Equation compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Metal carbonates decompose when heated. Lead carbonate → lead oxide + carbon dioxide. The aim of this experiment is to compare the reactivity of some different metal. State the type of reactions for the. Some. Lead Carbonate Thermal Decomposition Word Equation.
From www.youtube.com
Standard Reactions Thermal of a metal carbonate YouTube Lead Carbonate Thermal Decomposition Word Equation Revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. consider the following word equation for a thermal decomposition reaction: Some carbonates are more reactive than others. . Lead Carbonate Thermal Decomposition Word Equation.
From learninglibrarythompson.z13.web.core.windows.net
Which Reaction Is A Reaction Lead Carbonate Thermal Decomposition Word Equation The aim of this experiment is to compare the reactivity of some different metal. Metal carbonates decompose when heated. in this video, we will learn how to describe and give examples of thermal decomposition reactions. the word equation for this reaction is written like this: consider the following word equation for a thermal decomposition reaction: Lead carbonate. Lead Carbonate Thermal Decomposition Word Equation.
From studylib.net
Thermal Lead Carbonate Thermal Decomposition Word Equation Copper carbonate produces copper oxide, plus carbon. State the type of reactions for the. Revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry. in this video, we will learn how to describe and give examples of thermal decomposition reactions. compare the thermal stabilities of carbonates of reactive metals, like sodium and. Lead Carbonate Thermal Decomposition Word Equation.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID6307470 Lead Carbonate Thermal Decomposition Word Equation compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Lead carbonate → lead oxide + carbon dioxide. The aim of this experiment is to compare the reactivity of some different metal. Metal carbonates decompose when heated. in this video, we will learn how. Lead Carbonate Thermal Decomposition Word Equation.
From www.numerade.com
SOLVED Part Write a balanced equation for the of lead Lead Carbonate Thermal Decomposition Word Equation consider the following word equation for a thermal decomposition reaction: compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. The aim of this experiment is to compare the reactivity of some different metal. Metal carbonates decompose when heated. in this video, we. Lead Carbonate Thermal Decomposition Word Equation.
From exoxkmyoc.blob.core.windows.net
Thermal Of Lead Iv Oxide at James Dewey blog Lead Carbonate Thermal Decomposition Word Equation consider the following word equation for a thermal decomposition reaction: The aim of this experiment is to compare the reactivity of some different metal. Revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry. compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less. Lead Carbonate Thermal Decomposition Word Equation.
From www.gauthmath.com
Solved The chemical equation for the thermal of calcium Lead Carbonate Thermal Decomposition Word Equation compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Lead carbonate → lead oxide + carbon dioxide. We’ll explain why thermal decomposition is. the word equation for this reaction is written like this: consider the following word equation for a thermal decomposition. Lead Carbonate Thermal Decomposition Word Equation.