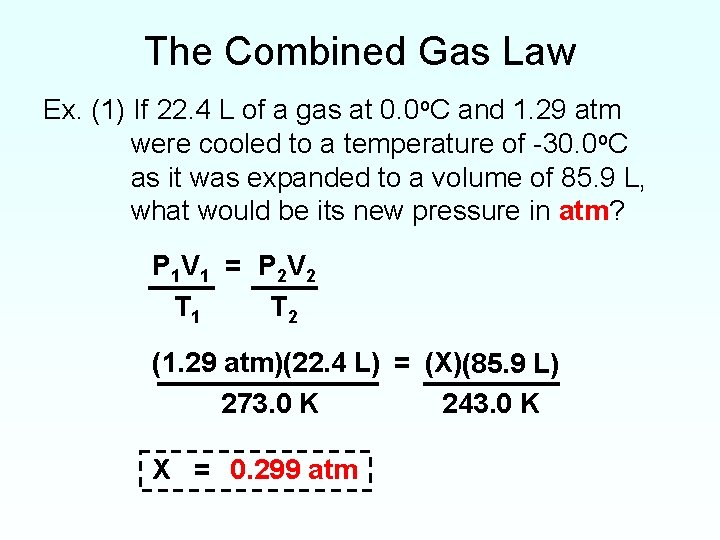
Combined Gas Law Equations . Pvt= k (k being the proportionality constant) the combined gas law combines. What is the combined gas law? The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas. The combined gas law states that when the amount of gas is fixed, the product of pressure (p), volume (v), and temperature (t) is equal to a constant (k). It can be used to calculate any of the four. It is derived from three other names gas laws,. In this section you will learn to use the combined gas law equation, including how to use it as a replacement for all of the individual gas laws. The combined gas law defines the relationship between pressure, temperature, and volume. The combined gas law states that for a fixed amount of gas: The combined gas law is formed by combining four different laws.
from
It can be used to calculate any of the four. In this section you will learn to use the combined gas law equation, including how to use it as a replacement for all of the individual gas laws. The combined gas law states that when the amount of gas is fixed, the product of pressure (p), volume (v), and temperature (t) is equal to a constant (k). The combined gas law states that for a fixed amount of gas: The combined gas law is formed by combining four different laws. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas. What is the combined gas law? The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; It is derived from three other names gas laws,. Pvt= k (k being the proportionality constant) the combined gas law combines.
Combined Gas Law Equations The combined gas law defines the relationship between pressure, temperature, and volume. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; It is derived from three other names gas laws,. The combined gas law states that for a fixed amount of gas: What is the combined gas law? Pvt= k (k being the proportionality constant) the combined gas law combines. The combined gas law is formed by combining four different laws. The combined gas law states that when the amount of gas is fixed, the product of pressure (p), volume (v), and temperature (t) is equal to a constant (k). The combined gas law defines the relationship between pressure, temperature, and volume. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas. In this section you will learn to use the combined gas law equation, including how to use it as a replacement for all of the individual gas laws. It can be used to calculate any of the four.
From
Combined Gas Law Equations It can be used to calculate any of the four. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas. Pvt= k (k being the proportionality constant) the combined gas law combines. The combined gas law defines the relationship between pressure, temperature, and volume. The combined gas law states that. Combined Gas Law Equations.
From www.slideserve.com
PPT The Gas Laws PowerPoint Presentation, free download ID3358137 Combined Gas Law Equations The combined gas law states that when the amount of gas is fixed, the product of pressure (p), volume (v), and temperature (t) is equal to a constant (k). It is derived from three other names gas laws,. It can be used to calculate any of the four. The combined gas law states that for a fixed amount of gas:. Combined Gas Law Equations.
From
Combined Gas Law Equations What is the combined gas law? The combined gas law states that when the amount of gas is fixed, the product of pressure (p), volume (v), and temperature (t) is equal to a constant (k). The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; In. Combined Gas Law Equations.
From inspiritvr.com
Combined Gas Law Study Guide Inspirit Learning Inc Combined Gas Law Equations The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; It can be used to calculate any of the four. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas. The combined gas law states that. Combined Gas Law Equations.
From
Combined Gas Law Equations The combined gas law defines the relationship between pressure, temperature, and volume. The combined gas law is formed by combining four different laws. It can be used to calculate any of the four. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas. In this section you will learn to. Combined Gas Law Equations.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation Combined Gas Law Equations In this section you will learn to use the combined gas law equation, including how to use it as a replacement for all of the individual gas laws. What is the combined gas law? It is derived from three other names gas laws,. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and. Combined Gas Law Equations.
From
Combined Gas Law Equations The combined gas law states that when the amount of gas is fixed, the product of pressure (p), volume (v), and temperature (t) is equal to a constant (k). The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; The combined gas law is formed by. Combined Gas Law Equations.
From
Combined Gas Law Equations It can be used to calculate any of the four. What is the combined gas law? It is derived from three other names gas laws,. The combined gas law states that for a fixed amount of gas: The combined gas law states that when the amount of gas is fixed, the product of pressure (p), volume (v), and temperature (t). Combined Gas Law Equations.
From
Combined Gas Law Equations The combined gas law defines the relationship between pressure, temperature, and volume. The combined gas law is formed by combining four different laws. It is derived from three other names gas laws,. What is the combined gas law? It can be used to calculate any of the four. The combined gas law states that for a fixed amount of gas:. Combined Gas Law Equations.
From www.youtube.com
Ideal Gas Laws 2 The Combined Gas Equation YouTube Combined Gas Law Equations It is derived from three other names gas laws,. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; The combined gas law is formed by combining four different laws. The combined gas law defines the relationship between pressure, temperature, and volume. In this section you. Combined Gas Law Equations.
From
Combined Gas Law Equations The combined gas law is formed by combining four different laws. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas. The combined gas law states that for a fixed amount of gas: Pvt= k (k being the proportionality constant) the combined gas law combines. The ideal gas law is. Combined Gas Law Equations.
From
Combined Gas Law Equations The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; The combined gas law is formed by combining four different laws. The combined gas law defines the relationship between pressure, temperature, and volume. What is the combined gas law? The combined gas law states that for. Combined Gas Law Equations.
From
Combined Gas Law Equations It is derived from three other names gas laws,. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; The combined gas law is formed by combining four different laws. It can be used to calculate any of the four. The combined gas law states that. Combined Gas Law Equations.
From
Combined Gas Law Equations The combined gas law defines the relationship between pressure, temperature, and volume. It is derived from three other names gas laws,. In this section you will learn to use the combined gas law equation, including how to use it as a replacement for all of the individual gas laws. What is the combined gas law? The combined gas law states. Combined Gas Law Equations.
From www.slideserve.com
PPT Properties of Gases & Gas Laws PowerPoint Presentation, free Combined Gas Law Equations It can be used to calculate any of the four. The combined gas law states that when the amount of gas is fixed, the product of pressure (p), volume (v), and temperature (t) is equal to a constant (k). What is the combined gas law? The combined gas law states that for a fixed amount of gas: Pvt= k (k. Combined Gas Law Equations.
From chemistryskills.com
Ideal Gas Law Combined Gas Law Chemistry Skills Combined Gas Law Equations The combined gas law states that when the amount of gas is fixed, the product of pressure (p), volume (v), and temperature (t) is equal to a constant (k). It can be used to calculate any of the four. The combined gas law states that for a fixed amount of gas: The combined gas law expresses the relationship between the. Combined Gas Law Equations.
From lessonlibcallahan.z19.web.core.windows.net
Combined Gas Law Explained Combined Gas Law Equations The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; It can be used to calculate any of the four. The combined gas law is formed by combining four different laws. The combined gas law states that for a fixed amount of gas: Pvt= k (k. Combined Gas Law Equations.
From
Combined Gas Law Equations It can be used to calculate any of the four. What is the combined gas law? Pvt= k (k being the proportionality constant) the combined gas law combines. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; The combined gas law defines the relationship between. Combined Gas Law Equations.
From
Combined Gas Law Equations It is derived from three other names gas laws,. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; The combined gas law is formed by combining four different laws. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a. Combined Gas Law Equations.
From
Combined Gas Law Equations The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; What is the combined gas law? The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas. Pvt= k (k being the proportionality constant) the combined gas. Combined Gas Law Equations.
From
Combined Gas Law Equations The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas. The combined gas law states that when the amount of gas is fixed, the product of pressure (p), volume (v), and temperature (t) is equal to a constant (k). In this section you will learn to use the combined gas. Combined Gas Law Equations.
From
Combined Gas Law Equations It can be used to calculate any of the four. The combined gas law defines the relationship between pressure, temperature, and volume. In this section you will learn to use the combined gas law equation, including how to use it as a replacement for all of the individual gas laws. The ideal gas law is derived from empirical relationships among. Combined Gas Law Equations.
From mmerevise.co.uk
The Ideal Gas Equation MME Combined Gas Law Equations The combined gas law defines the relationship between pressure, temperature, and volume. It can be used to calculate any of the four. The combined gas law is formed by combining four different laws. The combined gas law states that when the amount of gas is fixed, the product of pressure (p), volume (v), and temperature (t) is equal to a. Combined Gas Law Equations.
From
Combined Gas Law Equations The combined gas law defines the relationship between pressure, temperature, and volume. In this section you will learn to use the combined gas law equation, including how to use it as a replacement for all of the individual gas laws. The combined gas law states that for a fixed amount of gas: The combined gas law expresses the relationship between. Combined Gas Law Equations.
From
Combined Gas Law Equations What is the combined gas law? The combined gas law is formed by combining four different laws. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; It is derived from three other names gas laws,. Pvt= k (k being the proportionality constant) the combined gas. Combined Gas Law Equations.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation Combined Gas Law Equations The combined gas law defines the relationship between pressure, temperature, and volume. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas. In this section you will learn to use the combined gas law equation, including how to use it as a replacement for all of the individual gas laws.. Combined Gas Law Equations.
From www.sliderbase.com
Gas Laws Presentation Chemistry Combined Gas Law Equations It is derived from three other names gas laws,. The combined gas law is formed by combining four different laws. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; In this section you will learn to use the combined gas law equation, including how to. Combined Gas Law Equations.
From
Combined Gas Law Equations The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; Pvt= k (k being the proportionality constant) the combined gas law combines. It is derived from three other names gas laws,. In this section you will learn to use the combined gas law equation, including how. Combined Gas Law Equations.
From
Combined Gas Law Equations It can be used to calculate any of the four. What is the combined gas law? The combined gas law states that for a fixed amount of gas: In this section you will learn to use the combined gas law equation, including how to use it as a replacement for all of the individual gas laws. The ideal gas law. Combined Gas Law Equations.
From
Combined Gas Law Equations It can be used to calculate any of the four. The combined gas law states that when the amount of gas is fixed, the product of pressure (p), volume (v), and temperature (t) is equal to a constant (k). In this section you will learn to use the combined gas law equation, including how to use it as a replacement. Combined Gas Law Equations.
From
Combined Gas Law Equations The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; In this section you will learn to use the combined gas law equation, including how to use it as a replacement for all of the individual gas laws. What is the combined gas law? The combined. Combined Gas Law Equations.
From
Combined Gas Law Equations The combined gas law is formed by combining four different laws. In this section you will learn to use the combined gas law equation, including how to use it as a replacement for all of the individual gas laws. The combined gas law defines the relationship between pressure, temperature, and volume. The ideal gas law is derived from empirical relationships. Combined Gas Law Equations.
From www.showme.com
W34D4 combined gas law examples Science, Chemistry, Gases ShowMe Combined Gas Law Equations It is derived from three other names gas laws,. The combined gas law defines the relationship between pressure, temperature, and volume. Pvt= k (k being the proportionality constant) the combined gas law combines. The combined gas law is formed by combining four different laws. The combined gas law states that when the amount of gas is fixed, the product of. Combined Gas Law Equations.
From printablelistgather.z4.web.core.windows.net
Gas Law Formulas Sheet Combined Gas Law Equations The combined gas law is formed by combining four different laws. It can be used to calculate any of the four. The combined gas law states that when the amount of gas is fixed, the product of pressure (p), volume (v), and temperature (t) is equal to a constant (k). What is the combined gas law? The combined gas law. Combined Gas Law Equations.
From
Combined Gas Law Equations Pvt= k (k being the proportionality constant) the combined gas law combines. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas; It is derived from three other names gas laws,. In this section you will learn to use the combined gas law equation, including how. Combined Gas Law Equations.