How Do You Increase The Concentration Of A Reactant . increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. Increasing the concentration of one or more reactants will often increase the rate of reaction. a → productsr = k[a]m. for many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction. Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. This page explains why changing the concentration changes reaction rates, and. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. the effect of concentration on the rates of chemical reactions. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,.
from www.chegg.com
increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. for many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction. a → productsr = k[a]m. Increasing the concentration of one or more reactants will often increase the rate of reaction. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. the effect of concentration on the rates of chemical reactions. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. This page explains why changing the concentration changes reaction rates, and.
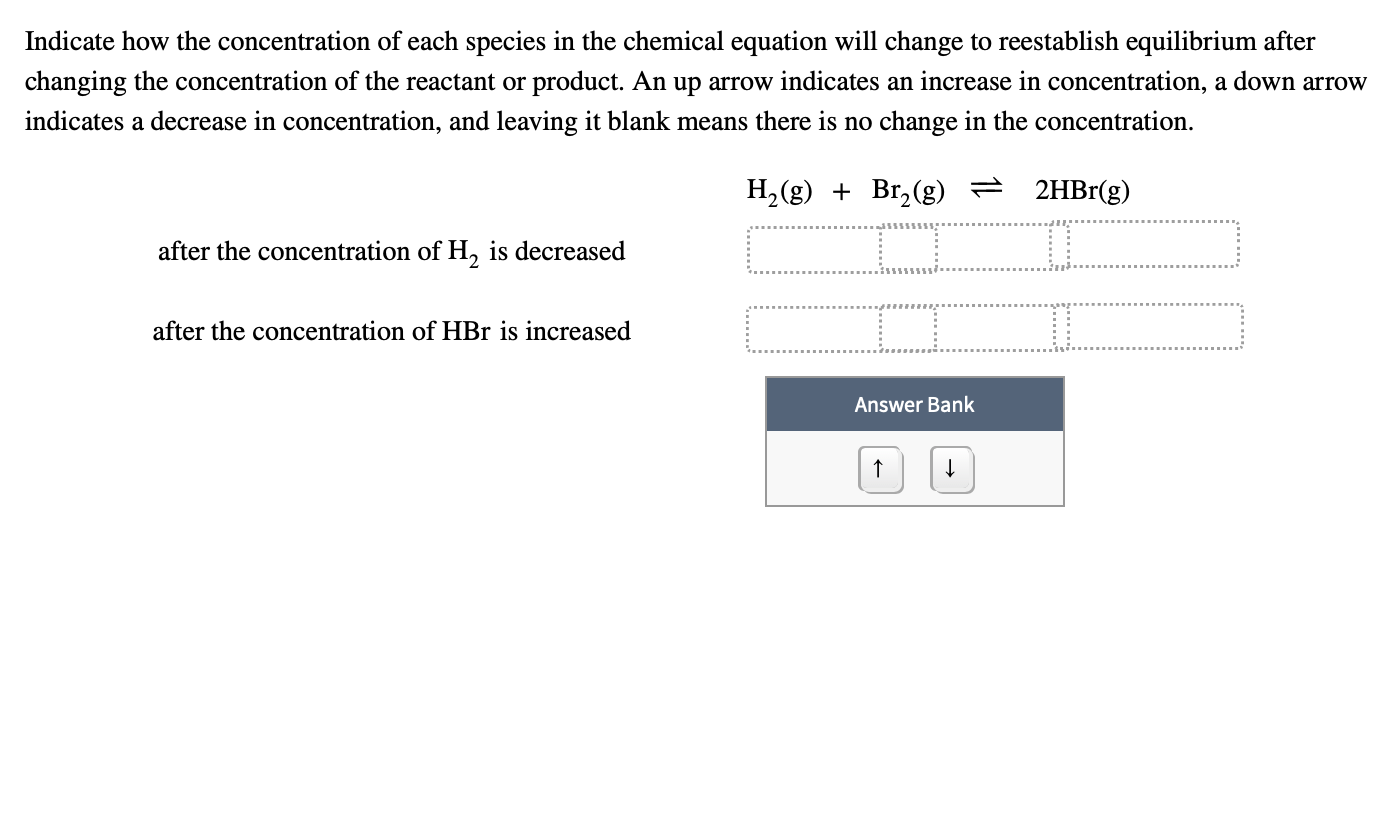
Solved Indicate how the concentration of each species in the
How Do You Increase The Concentration Of A Reactant Increasing the concentration of one or more reactants will often increase the rate of reaction. for many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction. This page explains why changing the concentration changes reaction rates, and. the effect of concentration on the rates of chemical reactions. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. a → productsr = k[a]m. Increasing the concentration of one or more reactants will often increase the rate of reaction. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant.
From slideplayer.com
Shifting Equilibrium. ppt download How Do You Increase The Concentration Of A Reactant a → productsr = k[a]m. Increasing the concentration of one or more reactants will often increase the rate of reaction. Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. This page explains why changing the concentration changes reaction rates, and. for many reactions involving liquids or gases, increasing the. How Do You Increase The Concentration Of A Reactant.
From giohwmanr.blob.core.windows.net
Can A Catalyst Increase The Concentration Of The Reactants at Nancy How Do You Increase The Concentration Of A Reactant for many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction. Increasing the concentration of one or more reactants will often increase the rate of reaction. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. the effect of concentration. How Do You Increase The Concentration Of A Reactant.
From www.nagwa.com
Question Video Identifying the Effect of Increased Reactant How Do You Increase The Concentration Of A Reactant Increasing the concentration of one or more reactants will often increase the rate of reaction. for many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction. Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. the effect of concentration on the rates. How Do You Increase The Concentration Of A Reactant.
From www.gauthmath.com
Solved How does changing concentration effect the rate of a chemical How Do You Increase The Concentration Of A Reactant Increasing the concentration of one or more reactants will often increase the rate of reaction. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. for many reactions involving liquids or gases,. How Do You Increase The Concentration Of A Reactant.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID6614655 How Do You Increase The Concentration Of A Reactant the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. Increasing the concentration of one or more reactants will often increase the rate of reaction. the effect of concentration on the rates of chemical reactions. increasing the concentration of a reactant increases the frequency of collisions between. How Do You Increase The Concentration Of A Reactant.
From www.slideserve.com
PPT Rates of Reaction PowerPoint Presentation, free download ID2689768 How Do You Increase The Concentration Of A Reactant increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. This page explains why changing the concentration changes reaction rates, and. the rate of a chemical reaction can be changed by. How Do You Increase The Concentration Of A Reactant.
From thewrightinitiative.com
Consider the reaction How Do You Increase The Concentration Of A Reactant a → productsr = k[a]m. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. Increasing the concentration of one or more reactants will often increase the rate of reaction. . How Do You Increase The Concentration Of A Reactant.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID How Do You Increase The Concentration Of A Reactant increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. the effect of concentration on the rates of chemical reactions. Increasing the concentration of one or more reactants will often increase the rate of reaction. the rate of a chemical reaction can be changed by altering the concentration of a reactant in. How Do You Increase The Concentration Of A Reactant.
From giohwmanr.blob.core.windows.net
Can A Catalyst Increase The Concentration Of The Reactants at Nancy How Do You Increase The Concentration Of A Reactant the effect of concentration on the rates of chemical reactions. Increasing the concentration of one or more reactants will often increase the rate of reaction. Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. the rate of a chemical reaction can be changed by altering the concentration of a. How Do You Increase The Concentration Of A Reactant.
From www.nagwa.com
Question Video Identifying Which Statement Regarding the Effect of How Do You Increase The Concentration Of A Reactant the effect of concentration on the rates of chemical reactions. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. This page explains why changing the concentration changes reaction rates, and. for many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate. How Do You Increase The Concentration Of A Reactant.
From chemnotcheem.com
5 ways to increase reaction speed Chem Not Cheem How Do You Increase The Concentration Of A Reactant This page explains why changing the concentration changes reaction rates, and. Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. the rate constant (k) of a rate law is a constant of proportionality between. How Do You Increase The Concentration Of A Reactant.
From sciencenotes.org
What Is a Reactant in Chemistry? Definition and Examples How Do You Increase The Concentration Of A Reactant Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. the effect of concentration on the rates of chemical reactions. This page explains why changing the concentration changes reaction rates, and. the rate constant. How Do You Increase The Concentration Of A Reactant.
From www.youtube.com
Reaction Rates Demonstration Concentration Change YouTube How Do You Increase The Concentration Of A Reactant increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. for many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction. This page explains why changing the concentration changes reaction rates, and. increasing the concentration of a reactant increases the frequency of collisions between. How Do You Increase The Concentration Of A Reactant.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent How Do You Increase The Concentration Of A Reactant Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. a → productsr = k[a]m. Increasing the concentration of one or more reactants will often increase the rate of reaction. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant.. How Do You Increase The Concentration Of A Reactant.
From www.youtube.com
Finding Concentration of each substance at equilibrium YouTube How Do You Increase The Concentration Of A Reactant increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. This page explains why changing the concentration changes reaction rates, and. the effect of concentration on the rates of chemical reactions. a → productsr = k[a]m. Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction,. How Do You Increase The Concentration Of A Reactant.
From www.youtube.com
In a first order reaction the concentration of the reactant is reduced How Do You Increase The Concentration Of A Reactant the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. This page explains why changing the concentration changes reaction rates, and. Increasing the concentration of one or more reactants will often. How Do You Increase The Concentration Of A Reactant.
From www.numerade.com
Text Indicate how the concentration of each species in the chemical How Do You Increase The Concentration Of A Reactant Increasing the concentration of one or more reactants will often increase the rate of reaction. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. Using the convention introduced in section 14.2.1. How Do You Increase The Concentration Of A Reactant.
From www.chegg.com
Solved Indicate how the concentration of each aqueous How Do You Increase The Concentration Of A Reactant Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. This page explains why changing the concentration changes reaction rates, and. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. for many reactions involving liquids or gases, increasing the concentration of the reactants. How Do You Increase The Concentration Of A Reactant.
From www.pinterest.co.uk
Rate of reaction & concentration. Aqa chemistry, Additional science How Do You Increase The Concentration Of A Reactant Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. for many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction. the effect of concentration on the rates of chemical reactions. increasing the concentration of a reactant increases the frequency of collisions. How Do You Increase The Concentration Of A Reactant.
From www.toppr.com
In which of the following reactions, the concentration of reactant is How Do You Increase The Concentration Of A Reactant the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. the effect of concentration on the rates of chemical reactions. a → productsr = k[a]m. . How Do You Increase The Concentration Of A Reactant.
From www.youtube.com
How does concentration affect rate of reaction? YouTube How Do You Increase The Concentration Of A Reactant Increasing the concentration of one or more reactants will often increase the rate of reaction. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. This page explains why changing the concentration changes. How Do You Increase The Concentration Of A Reactant.
From www.slideserve.com
PPT Rates of Reaction PowerPoint Presentation, free download ID4284871 How Do You Increase The Concentration Of A Reactant for many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. the effect of concentration on the rates. How Do You Increase The Concentration Of A Reactant.
From www.slideserve.com
PPT What does rate of reaction mean? PowerPoint Presentation, free How Do You Increase The Concentration Of A Reactant increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. for many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction. the effect of concentration on the rates of chemical reactions. This page explains why changing the concentration changes reaction rates, and. a. How Do You Increase The Concentration Of A Reactant.
From hxebyxmkl.blob.core.windows.net
How Does Presence Of Catalyst Affect The Rate Of Reaction at Kristi How Do You Increase The Concentration Of A Reactant the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. the rate of. How Do You Increase The Concentration Of A Reactant.
From www.expii.com
Concentration (Enzyme Reaction Rates) — Effects & Role Expii How Do You Increase The Concentration Of A Reactant Increasing the concentration of one or more reactants will often increase the rate of reaction. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. increasing the. How Do You Increase The Concentration Of A Reactant.
From www.chegg.com
Solved Indicate how the concentration of each species in the How Do You Increase The Concentration Of A Reactant the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. Increasing the concentration of one or more reactants will often increase the rate of reaction. Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. the effect of concentration on. How Do You Increase The Concentration Of A Reactant.
From fyoxbkinz.blob.core.windows.net
Enzymes Increase The Rate Of A Reaction By Which Contributing Factor at How Do You Increase The Concentration Of A Reactant a → productsr = k[a]m. Increasing the concentration of one or more reactants will often increase the rate of reaction. Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. the effect of concentration. How Do You Increase The Concentration Of A Reactant.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science How Do You Increase The Concentration Of A Reactant This page explains why changing the concentration changes reaction rates, and. Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. a → productsr = k[a]m. for many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction. increasing the concentration of a. How Do You Increase The Concentration Of A Reactant.
From slideplayer.com
Unit 6 Solutions and ppt download How Do You Increase The Concentration Of A Reactant This page explains why changing the concentration changes reaction rates, and. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. Increasing the concentration of one or more reactants will often increase the rate of reaction. the rate constant (k) of a rate law is a constant of. How Do You Increase The Concentration Of A Reactant.
From needchemistry247.weebly.com
India Need Chemistry 24 7 How Do You Increase The Concentration Of A Reactant increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. This page explains why changing the concentration changes reaction rates, and. the effect of concentration on the rates of chemical reactions. Increasing the concentration of one. How Do You Increase The Concentration Of A Reactant.
From www.slideserve.com
PPT Chapter 17 reaction rates PowerPoint Presentation, free download How Do You Increase The Concentration Of A Reactant Increasing the concentration of one or more reactants will often increase the rate of reaction. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. for many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction. the rate of a. How Do You Increase The Concentration Of A Reactant.
From www.slideserve.com
PPT IB Chemistry PowerPoint Presentation, free download ID2738432 How Do You Increase The Concentration Of A Reactant Using the convention introduced in section 14.2.1 of this chapter, r is the rate of reaction, [a]represent the. a → productsr = k[a]m. Increasing the concentration of one or more reactants will often increase the rate of reaction. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the.. How Do You Increase The Concentration Of A Reactant.
From socratic.org
What determines when a system reaches equilibrium? What observations How Do You Increase The Concentration Of A Reactant increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. for many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction. the effect of concentration on the rates of chemical reactions. Using the convention introduced in section 14.2.1 of this chapter, r is the. How Do You Increase The Concentration Of A Reactant.
From www.youtube.com
In a reaction, when the concentration of reactant is increased two How Do You Increase The Concentration Of A Reactant the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. This page explains why changing the concentration changes reaction rates, and. for many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction. Increasing the concentration of one or more reactants will. How Do You Increase The Concentration Of A Reactant.
From slideplayer.com
Do Now Write the Kc expression for the following ppt download How Do You Increase The Concentration Of A Reactant This page explains why changing the concentration changes reaction rates, and. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. for many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction. the effect of concentration on the rates of chemical reactions. increasing. How Do You Increase The Concentration Of A Reactant.