Titration Of Kmno4 With Feso4 . The solution was colourless, then. In close proximity to the endpoint, the action. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. In these titrations fe2+ is. i recently did an experiment at school, where i had to titrate kmno4 with feso4. redox titrations can be used to find the amount of iron in a sample (or other reagents).
from www.chegg.com
i recently did an experiment at school, where i had to titrate kmno4 with feso4. In close proximity to the endpoint, the action. this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. In these titrations fe2+ is. The solution was colourless, then. redox titrations can be used to find the amount of iron in a sample (or other reagents). In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of.
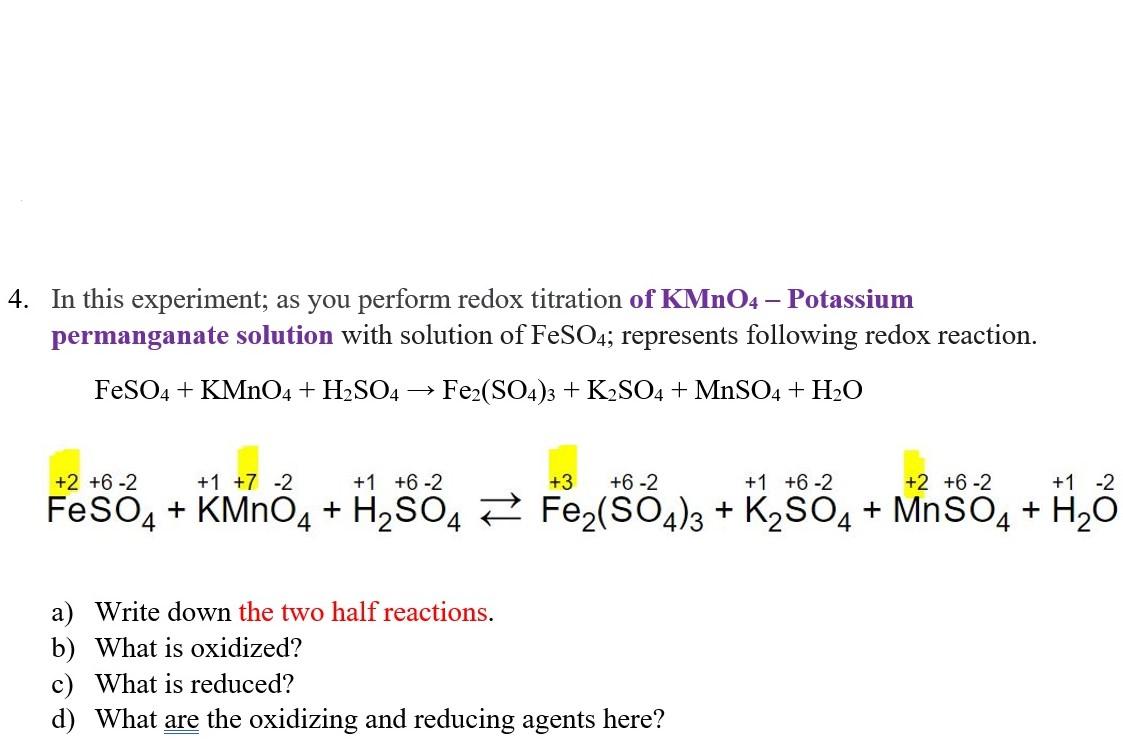
Solved 4. In this experiment; as you perform redox titration
Titration Of Kmno4 With Feso4 redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. redox titrations can be used to find the amount of iron in a sample (or other reagents). The solution was colourless, then. In these titrations fe2+ is. i recently did an experiment at school, where i had to titrate kmno4 with feso4. this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. In close proximity to the endpoint, the action. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an.
From www.numerade.com
SOLVED Text LESSON Redox Titration Lab Titration Redox Titration Titration Of Kmno4 With Feso4 i recently did an experiment at school, where i had to titrate kmno4 with feso4. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. redox titration of ferrous sulphate (fe+2). Titration Of Kmno4 With Feso4.
From www.youtube.com
KMnO4 Redox Titration With FeSO4.7H20 YouTube Titration Of Kmno4 With Feso4 The solution was colourless, then. i recently did an experiment at school, where i had to titrate kmno4 with feso4. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. In close proximity to the endpoint, the action. the titration of potassium permanganate (kmno 4) against mohr salt. Titration Of Kmno4 With Feso4.
From www.chegg.com
Solved KMnO4 used (mL)=10.87 mL Beginning with your mL of Titration Of Kmno4 With Feso4 redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. In these titrations fe2+ is. this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. The solution was colourless, then. in the redox. Titration Of Kmno4 With Feso4.
From exybsrswn.blob.core.windows.net
Redox Titration Kmno4 at Amanda Young blog Titration Of Kmno4 With Feso4 In close proximity to the endpoint, the action. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. i recently did an experiment at school, where i had to titrate kmno4 with feso4. the titration of potassium permanganate (kmno. Titration Of Kmno4 With Feso4.
From www.youtube.com
titration of kmno4 with mohr's salt class 12 determine the Titration Of Kmno4 With Feso4 In close proximity to the endpoint, the action. this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. redox titrations can be used to find the amount of iron in a sample (or other reagents). the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. The solution. Titration Of Kmno4 With Feso4.
From chemistrypubs.com
Titration of Oxalic Acid with KMnO4 Chemistrupubs Titration Of Kmno4 With Feso4 The solution was colourless, then. In close proximity to the endpoint, the action. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. i recently did an experiment at school, where. Titration Of Kmno4 With Feso4.
From www.reddit.com
Oxidation and reduction help. Here's the question The titration of an Titration Of Kmno4 With Feso4 In close proximity to the endpoint, the action. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. . Titration Of Kmno4 With Feso4.
From www.youtube.com
Titration of Kmno4 against Oxalic Acid YouTube Titration Of Kmno4 With Feso4 The solution was colourless, then. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. In these titrations fe2+ is. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. redox titrations can be used to find the amount of iron in a sample (or. Titration Of Kmno4 With Feso4.
From www.reddit.com
Why does the FeSO4 (added with H2SO4) titrated with KMnO4 slowly Titration Of Kmno4 With Feso4 The solution was colourless, then. In close proximity to the endpoint, the action. this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. redox titrations can be used to find the amount of iron in a sample (or other reagents). in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the. Titration Of Kmno4 With Feso4.
From www.numerade.com
SOLVED Experiment Na UZ Experiment Nume Estimation of ferrous ions (Fe Titration Of Kmno4 With Feso4 i recently did an experiment at school, where i had to titrate kmno4 with feso4. In these titrations fe2+ is. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution. Titration Of Kmno4 With Feso4.
From www.meritnation.com
a) In the titration of FeSO4 with KMnO4 in the acidic medium, why is Titration Of Kmno4 With Feso4 redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. redox titrations can be. Titration Of Kmno4 With Feso4.
From www.youtube.com
Titration KMnO4 Vs Mohr Salt in Hindi Full Experiment Titration Of Kmno4 With Feso4 The solution was colourless, then. In close proximity to the endpoint, the action. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. redox titrations can be used. Titration Of Kmno4 With Feso4.
From studylib.net
Expt 2Redox titration Titration Of Kmno4 With Feso4 redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. redox titrations can be used to find the amount of iron in a sample (or other reagents). The solution was colourless, then. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in. Titration Of Kmno4 With Feso4.
From www.vrogue.co
Suppose That 22 65 Ml Of A 0 02024 M Permanganate Sol vrogue.co Titration Of Kmno4 With Feso4 i recently did an experiment at school, where i had to titrate kmno4 with feso4. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. The solution was colourless, then. In these titrations fe2+ is. redox titrations can be used to find the amount of iron in a. Titration Of Kmno4 With Feso4.
From www.youtube.com
Determination of Concentration of KMnO4 Soution using Ferrous Ammonium Titration Of Kmno4 With Feso4 In these titrations fe2+ is. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe. Titration Of Kmno4 With Feso4.
From praxilabs.com
Standardization of Potassium Permanganate In 7 Steps Titration Of Kmno4 With Feso4 this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. The solution was colourless, then. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in. Titration Of Kmno4 With Feso4.
From www.numerade.com
SOLVED What mass of FeSO4.(NH4)2SO4. 6H2O is present in a sample if it Titration Of Kmno4 With Feso4 in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. The solution was colourless, then. this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. the titration of potassium permanganate. Titration Of Kmno4 With Feso4.
From www.youtube.com
Redox titration potassium permanganate ferrous sulphate KMnO4 and Titration Of Kmno4 With Feso4 redox titrations can be used to find the amount of iron in a sample (or other reagents). i recently did an experiment at school, where i had to titrate kmno4 with feso4. In close proximity to the endpoint, the action. The solution was colourless, then. this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution. Titration Of Kmno4 With Feso4.
From xaydungso.vn
Phản Ứng Hóa Học H2SO4 + KMnO4 + FeSO4 Cân Bằng và Ứng Dụng Titration Of Kmno4 With Feso4 i recently did an experiment at school, where i had to titrate kmno4 with feso4. this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. In close proximity to the. Titration Of Kmno4 With Feso4.
From androidtraining.metmans.edu.eg
FeSO4 KMnO4 H2SO4 Fe2(SO4)3 MnSO4 H2O K2SO4 Tuition Tube, 42 OFF Titration Of Kmno4 With Feso4 in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. i recently did an experiment at school, where i had to titrate kmno4 with feso4.. Titration Of Kmno4 With Feso4.
From www.youtube.com
Titration Of Kmno4 Vs Oxalic Acid The Ultimate Guide Titration Titration Of Kmno4 With Feso4 in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. i recently did an experiment at school, where i had to titrate kmno4 with feso4. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. The solution was colourless, then. this. Titration Of Kmno4 With Feso4.
From www.youtube.com
Titration of class 12 th KMnO4 against FeSO4 solution YouTube Titration Of Kmno4 With Feso4 In these titrations fe2+ is. i recently did an experiment at school, where i had to titrate kmno4 with feso4. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. In close. Titration Of Kmno4 With Feso4.
From www.slideserve.com
PPT Balancing Redox Reactions Carol Brown Saint Mary’s Hall Titration Of Kmno4 With Feso4 in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. redox titrations can be used to find the amount of iron in a sample (or other reagents). In close proximity to the endpoint, the. Titration Of Kmno4 With Feso4.
From www.chegg.com
Solved 4. In this experiment; as you perform redox titration Titration Of Kmno4 With Feso4 In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. In close proximity to the endpoint, the action. redox titrations can be used to find the amount of iron in a sample (or other reagents). In these titrations fe2+ is. i recently did an. Titration Of Kmno4 With Feso4.
From unacademy.com
Mohr salt titration Titration Of Kmno4 With Feso4 In close proximity to the endpoint, the action. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. the titration of potassium permanganate (kmno 4) against mohr salt is an. Titration Of Kmno4 With Feso4.
From www.studypool.com
SOLUTION Redox titration Studypool Titration Of Kmno4 With Feso4 In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. i recently did an experiment at school, where i had to titrate kmno4 with feso4. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is.. Titration Of Kmno4 With Feso4.
From www.youtube.com
Redox Titration between MnO4 and Fe2+ YouTube Titration Of Kmno4 With Feso4 In these titrations fe2+ is. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. redox titrations can be used to find the amount of iron in a sample (or other reagents). this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. In close proximity to the endpoint, the action.. Titration Of Kmno4 With Feso4.
From www.toppr.com
When KMnO4 is titrated against FeSO4 (NH4)2SO4.6H2O the equivalent mass Titration Of Kmno4 With Feso4 in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. the. Titration Of Kmno4 With Feso4.
From brunofuga.adv.br
What Happens When KMnO4 Reacts With FeSO4? Will KMnO4 Get, 46 OFF Titration Of Kmno4 With Feso4 The solution was colourless, then. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. In close proximity to the endpoint, the action. this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution. Titration Of Kmno4 With Feso4.
From www.meritnation.com
20ml of feso4 7h2o requires 10 ml of 0 1 M kmno4 for complete titration Titration Of Kmno4 With Feso4 this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. redox titrations can be used to find the amount of iron in a sample (or other reagents). In this experiment you will use a. Titration Of Kmno4 With Feso4.
From www.youtube.com
KMnO4 and FeSO4.7H2O Redox Titration YouTube Titration Of Kmno4 With Feso4 this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. i recently did an experiment at school, where i had to titrate kmno4 with feso4. In close proximity to the. Titration Of Kmno4 With Feso4.
From www.youtube.com
Standardization of KMnO4 Redox Titration KMnO4 Vs Fe2+ Mohrs salt Titration Of Kmno4 With Feso4 redox titrations can be used to find the amount of iron in a sample (or other reagents). this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. i recently did an experiment at school, where i had to titrate kmno4 with feso4. The solution was colourless, then. In close proximity to the endpoint,. Titration Of Kmno4 With Feso4.
From www.youtube.com
Redox titraton of FeSO4 with K2Cr2O7 & calculation of equivalance point Titration Of Kmno4 With Feso4 i recently did an experiment at school, where i had to titrate kmno4 with feso4. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. The solution was colourless, then. In close proximity to the endpoint, the action. redox titrations can be used to find the amount of. Titration Of Kmno4 With Feso4.
From www.youtube.com
Redox titration of Fe+2 (FeSO4) with Ce+4 Ce(SO4)2 and calculation of Titration Of Kmno4 With Feso4 redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. In these titrations fe2+ is. this video demonstrates the redox titration between 0.02m potassium permanganate (kmno4) solution and iron. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. in. Titration Of Kmno4 With Feso4.
From www.numerade.com
SOLVED 100 ml of given KMnO4 solution titrates 10 ml of 0.1 m oxalic Titration Of Kmno4 With Feso4 in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. In these titrations fe2+ is. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of. In close proximity to the endpoint, the action. the titration of potassium permanganate (kmno 4) against mohr salt is an. Titration Of Kmno4 With Feso4.