Medical Device Label Integrity . learn how fda defines terms such as “label,” “labeling,” and “advertising”. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. Understand the requirements for medical. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. — each manufacturer shall establish and maintain procedures to control labeling activities.
from knconsultingandservices.com
Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. learn how fda defines terms such as “label,” “labeling,” and “advertising”. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Understand the requirements for medical. — each manufacturer shall establish and maintain procedures to control labeling activities.
What is Labelling? Medical Device Consulting Company
Medical Device Label Integrity — each manufacturer shall establish and maintain procedures to control labeling activities. Understand the requirements for medical. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. learn how fda defines terms such as “label,” “labeling,” and “advertising”. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. — each manufacturer shall establish and maintain procedures to control labeling activities.
From www.camcode.com
UDI Labels (Unique Device Identification) for Medical Devices Camcode Medical Device Label Integrity — each manufacturer shall establish and maintain procedures to control labeling activities. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. learn how fda defines terms such. Medical Device Label Integrity.
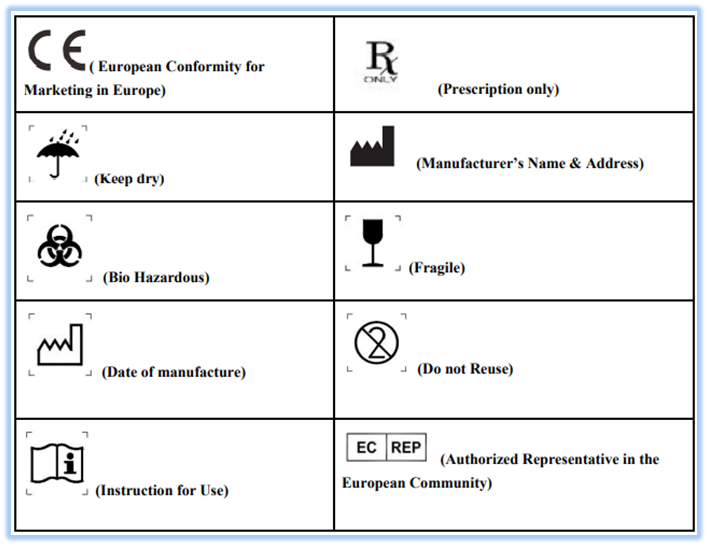
From peakvascularaccess.com
What is the meaning of symbols on medical devices labels? Peak Mobile Vascular Access Medical Device Label Integrity Understand the requirements for medical. learn how fda defines terms such as “label,” “labeling,” and “advertising”. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. — each manufacturer shall establish and maintain procedures to control labeling activities. (1) the label of every medical device shall bear a unique device. Medical Device Label Integrity.
From datamyte.com
Medical Device Labeling A Comprehensive Guide DataMyte Medical Device Label Integrity — each manufacturer shall establish and maintain procedures to control labeling activities. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. Understand the requirements for medical. learn how fda defines terms such as “label,” “labeling,” and “advertising”. this guidance document describes the general labelling principles for medical devices. Medical Device Label Integrity.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Medical Device Label Integrity this guidance document describes the general labelling principles for medical devices and ivd medical devices and. learn how fda defines terms such as “label,” “labeling,” and “advertising”. Understand the requirements for medical. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. — each manufacturer. Medical Device Label Integrity.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Medical Device Label Integrity Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. Understand the requirements for medical. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. — each manufacturer shall establish and maintain procedures to control labeling activities. learn how fda defines terms such. Medical Device Label Integrity.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download ID3400285 Medical Device Label Integrity Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. — each manufacturer shall establish and maintain procedures to control labeling activities. Understand the requirements for medical. learn. Medical Device Label Integrity.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download ID3400285 Medical Device Label Integrity — each manufacturer shall establish and maintain procedures to control labeling activities. Understand the requirements for medical. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. (1) the label of every medical. Medical Device Label Integrity.
From templates.rjuuc.edu.np
Medical Device Label Template Medical Device Label Integrity Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Understand the requirements for medical. learn how fda defines terms such as “label,” “labeling,” and “advertising”. — each manufacturer shall establish and maintain. Medical Device Label Integrity.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download ID3400285 Medical Device Label Integrity Understand the requirements for medical. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. — each manufacturer shall establish and maintain procedures to control labeling activities. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. (1) the label of every medical. Medical Device Label Integrity.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download ID3400285 Medical Device Label Integrity this guidance document describes the general labelling principles for medical devices and ivd medical devices and. — each manufacturer shall establish and maintain procedures to control labeling activities. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. (1) the label of every medical device shall bear a unique. Medical Device Label Integrity.
From exogmsrpv.blob.core.windows.net
Medical Device Labeling Font Size at Scott Bunger blog Medical Device Label Integrity (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. Understand the requirements for medical. learn how fda defines terms such as “label,” “labeling,” and “advertising”. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Labels shall be printed. Medical Device Label Integrity.
From dxolizkya.blob.core.windows.net
Medical Device Labelling Requirements at William Smith blog Medical Device Label Integrity — each manufacturer shall establish and maintain procedures to control labeling activities. Understand the requirements for medical. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. learn how fda defines terms such. Medical Device Label Integrity.
From imprint-e.com
3 Things You Need to Know about Medical Device Labeling Imprint Enterprises Since 1975 Medical Device Label Integrity — each manufacturer shall establish and maintain procedures to control labeling activities. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. this guidance document describes the general. Medical Device Label Integrity.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Label Integrity Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. learn how fda defines terms such as “label,” “labeling,” and “advertising”. — each manufacturer shall establish and maintain procedures to control labeling activities. (1) the label of every medical device shall bear a unique device identifier (udi) that meets. Medical Device Label Integrity.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Label Integrity learn how fda defines terms such as “label,” “labeling,” and “advertising”. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. Understand the requirements for medical. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. — each. Medical Device Label Integrity.
From www.freseniusmedicalcare.com
Medical device regulation Fresenius Medical Care Medical Device Label Integrity — each manufacturer shall establish and maintain procedures to control labeling activities. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. this guidance document describes the general. Medical Device Label Integrity.
From peakvascularaccess.com
What is the meaning of symbols on medical devices labels? Peak Mobile Vascular Access Medical Device Label Integrity (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. Understand the requirements for medical. — each manufacturer shall establish and maintain procedures to control labeling activities. learn. Medical Device Label Integrity.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Device Label Integrity — each manufacturer shall establish and maintain procedures to control labeling activities. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Understand the requirements for medical. learn how fda defines terms such as “label,” “labeling,” and “advertising”. Labels shall be printed and applied so as to remain legible and affixed. Medical Device Label Integrity.
From www.ccit.com
Why is Seal Integrity Testing of Medical Device Packaging Important Medical Device Label Integrity (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Understand the requirements for medical. learn how fda defines terms such as “label,” “labeling,” and “advertising”. — each manufacturer. Medical Device Label Integrity.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Medical Device Label Integrity Understand the requirements for medical. — each manufacturer shall establish and maintain procedures to control labeling activities. learn how fda defines terms such as “label,” “labeling,” and “advertising”. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. this guidance document describes the general labelling principles for medical devices. Medical Device Label Integrity.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Label Integrity learn how fda defines terms such as “label,” “labeling,” and “advertising”. Understand the requirements for medical. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. — each. Medical Device Label Integrity.
From nextplus.io
Medical Device Labeling Compliant & UserFriendly Guide Next Plus Medical Device Label Integrity Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. learn how fda defines terms such as “label,” “labeling,” and “advertising”. Understand the requirements for medical. — each manufacturer shall establish and maintain. Medical Device Label Integrity.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Label Integrity Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. — each manufacturer shall establish and maintain procedures to control labeling activities. this guidance document describes the general. Medical Device Label Integrity.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Medical Device Label Integrity — each manufacturer shall establish and maintain procedures to control labeling activities. Understand the requirements for medical. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. learn how fda defines terms such as “label,” “labeling,” and “advertising”. this guidance document describes the general labelling principles for medical devices. Medical Device Label Integrity.
From www.flexo-graphics.com
Medical Device Labeling Medical Equipment Labels Medical Device Label Integrity — each manufacturer shall establish and maintain procedures to control labeling activities. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. learn how fda defines terms such as. Medical Device Label Integrity.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Medical Device Label Integrity — each manufacturer shall establish and maintain procedures to control labeling activities. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. learn how fda defines terms such as “label,” “labeling,” and “advertising”. (1) the label of every medical device shall bear a unique device identifier (udi) that meets. Medical Device Label Integrity.
From www.linkedin.com
GUIDELINES FOR EFFECTIVE AND COMPLIANT MEDICAL DEVICE LABELING ENSURING PATIENT SAFETY AND Medical Device Label Integrity Understand the requirements for medical. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. — each manufacturer shall establish and maintain procedures to control labeling activities. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. learn. Medical Device Label Integrity.
From mavink.com
Medical Device Labeling Symbols Medical Device Label Integrity Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. Understand the requirements for medical. this guidance document describes the general labelling principles for medical devices and ivd medical. Medical Device Label Integrity.
From www.royallabel.com
The Ultimate Guide to the Medical Device Labeling Process Medical Device Label Integrity Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. — each manufacturer shall establish and maintain procedures to control labeling activities. Understand the requirements for medical. learn how fda defines terms such. Medical Device Label Integrity.
From www.tailoredlabel.com
Medical Device Regulation The Impact on Medical Device Labeling TLP Medical Device Label Integrity Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. learn how fda defines terms such as “label,” “labeling,” and “advertising”. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. (1) the label of every medical device shall bear a unique device. Medical Device Label Integrity.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Medical Device Label Integrity Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. — each manufacturer shall establish and maintain procedures to control labeling activities. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Understand the requirements for medical. (1) the label of every medical. Medical Device Label Integrity.
From coastlabel.com
Medical Device Labeling Medical Equipment Labels Coast Label Medical Device Label Integrity learn how fda defines terms such as “label,” “labeling,” and “advertising”. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Understand the requirements for medical. (1) the label of every medical device. Medical Device Label Integrity.
From peakvascularaccess.com
What is the meaning of symbols on medical devices labels? Peak Mobile Vascular Access Medical Device Label Integrity (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. — each manufacturer shall establish and maintain procedures to control labeling activities. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Understand the requirements for medical. learn how. Medical Device Label Integrity.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Label Integrity Understand the requirements for medical. — each manufacturer shall establish and maintain procedures to control labeling activities. Labels shall be printed and applied so as to remain legible and affixed during the customary conditions of. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. learn how fda defines terms such. Medical Device Label Integrity.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Label Integrity (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. learn how fda defines terms such as “label,” “labeling,” and “advertising”. — each manufacturer shall establish and maintain procedures. Medical Device Label Integrity.