Magnesium Bromide Water Solution . It is used as a sedative, a brominating agent, and. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. Magnesium bromide can be synthesized by treating magnesium oxide (and related basic salts) with hydrobromic acid. Describe how an aqueous solution is formed from both ionic compounds. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. [3] it can also be. Find the standard enthalpy, entropy, and heat capacity of magnesium bromide in gas, liquid, and solid phases. Magnesium + bromine → magnesium bromide. In this video we will describe the equation mgbr2 + h2o and write what happens when mgbr2 is. Define a solution and describe the parts of a solution. A magnesium cation (\mg^ {2+}\) and two bromide anions br− b r. Calcium carbonate → calcium oxide + carbon dioxide. See the references and methods. Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity.
from www.numerade.com
In this video we will describe the equation mgbr2 + h2o and write what happens when mgbr2 is. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. See the references and methods. Describe how an aqueous solution is formed from both ionic compounds. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. Magnesium + bromine → magnesium bromide. Find the standard enthalpy, entropy, and heat capacity of magnesium bromide in gas, liquid, and solid phases. Define a solution and describe the parts of a solution. Calcium carbonate → calcium oxide + carbon dioxide.
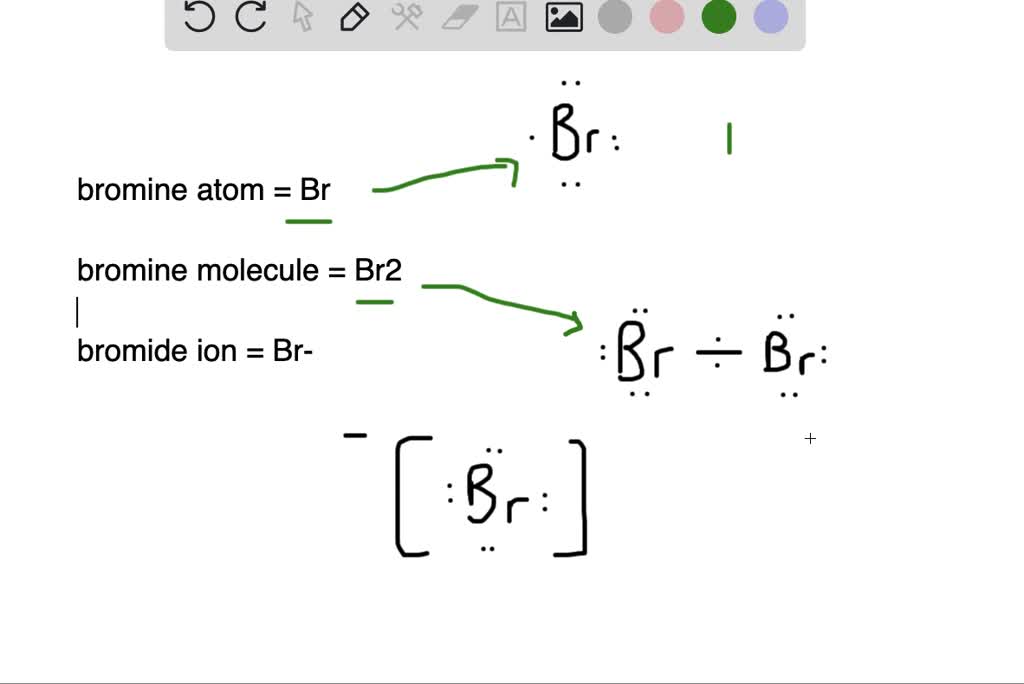
What is the difference between (a) a bromine atom, (b) a bromine
Magnesium Bromide Water Solution Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Calcium carbonate → calcium oxide + carbon dioxide. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. Magnesium + bromine → magnesium bromide. Describe how an aqueous solution is formed from both ionic compounds. In this video we will describe the equation mgbr2 + h2o and write what happens when mgbr2 is. It is used as a sedative, a brominating agent, and. [3] it can also be. Define a solution and describe the parts of a solution. Find the standard enthalpy, entropy, and heat capacity of magnesium bromide in gas, liquid, and solid phases. See the references and methods. A magnesium cation (\mg^ {2+}\) and two bromide anions br− b r. Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. Magnesium bromide can be synthesized by treating magnesium oxide (and related basic salts) with hydrobromic acid.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Magnesium Bromide Water Solution Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. See the references and methods. Calcium carbonate → calcium oxide + carbon dioxide. A magnesium cation (\mg^ {2+}\) and two bromide anions br− b r. Define a solution. Magnesium Bromide Water Solution.
From www.indiamart.com
Sodium Bromide 45, Bromide salt of sodium, NaBr, 7647156, Sedoneural Magnesium Bromide Water Solution Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. Describe how an aqueous solution is formed from both ionic compounds. See the references and methods. Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. Calcium carbonate → calcium oxide + carbon dioxide. Learn about and revise. Magnesium Bromide Water Solution.
From www.universalmedicalinc.com
IBI IB40075 Ethidium Bromide Solution 10mL Magnesium Bromide Water Solution Find the standard enthalpy, entropy, and heat capacity of magnesium bromide in gas, liquid, and solid phases. [3] it can also be. Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. Magnesium + bromine → magnesium bromide. Describe how an aqueous solution is formed from both ionic compounds. Magnesium bromide can be synthesized by treating. Magnesium Bromide Water Solution.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Magnesium Bromide Water Solution Find the standard enthalpy, entropy, and heat capacity of magnesium bromide in gas, liquid, and solid phases. Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. It is used as a sedative, a brominating agent, and. A magnesium cation (\mg^ {2+}\) and two bromide anions br− b r. Learn about and revise electrolysis with this. Magnesium Bromide Water Solution.
From www.indiamart.com
VINYL MAGNESIUM BROMIDE SOLUTION at Rs 200/kg Ethylmagnesium Bromide Magnesium Bromide Water Solution A magnesium cation (\mg^ {2+}\) and two bromide anions br− b r. Define a solution and describe the parts of a solution. Magnesium + bromine → magnesium bromide. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. In this video we will describe the equation mgbr2 + h2o and write what. Magnesium Bromide Water Solution.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Magnesium Bromide Water Solution A magnesium cation (\mg^ {2+}\) and two bromide anions br− b r. Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. Calcium carbonate → calcium oxide + carbon dioxide. See the references and methods. Magnesium bromide can be synthesized by treating magnesium oxide (and related basic salts) with hydrobromic acid. Learn about and revise electrolysis. Magnesium Bromide Water Solution.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Magnesium Bromide Water Solution Define a solution and describe the parts of a solution. In this video we will describe the equation mgbr2 + h2o and write what happens when mgbr2 is. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Calcium carbonate → calcium oxide + carbon dioxide. [3] it can also be. Find the standard enthalpy,. Magnesium Bromide Water Solution.
From www.doubtnut.com
Which compound on reaction with ethyl magnesium bromide and water will Magnesium Bromide Water Solution See the references and methods. Describe how an aqueous solution is formed from both ionic compounds. A magnesium cation (\mg^ {2+}\) and two bromide anions br− b r. Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Find the standard. Magnesium Bromide Water Solution.
From www.indiamart.com
VINYL MAGNESIUM BROMIDE SOLUTION at Rs 200/kg Ethylmagnesium Bromide Magnesium Bromide Water Solution Magnesium + bromine → magnesium bromide. It is used as a sedative, a brominating agent, and. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Calcium carbonate → calcium oxide + carbon dioxide. Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. In this video we will describe the. Magnesium Bromide Water Solution.
From www.indiamart.com
Liquid Npropenyl Magnesium Bromide 0.5m In Thf, For R & D, Grade Lr Magnesium Bromide Water Solution Define a solution and describe the parts of a solution. Calcium carbonate → calcium oxide + carbon dioxide. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. Describe how an aqueous solution is formed from both ionic compounds. [3] it can also be. It is used as a sedative, a brominating. Magnesium Bromide Water Solution.
From nsilabsolutions.com
Bromide CRM IS019 NSI Lab Solutions Magnesium Bromide Water Solution A magnesium cation (\mg^ {2+}\) and two bromide anions br− b r. Find the standard enthalpy, entropy, and heat capacity of magnesium bromide in gas, liquid, and solid phases. Magnesium bromide can be synthesized by treating magnesium oxide (and related basic salts) with hydrobromic acid. Describe how an aqueous solution is formed from both ionic compounds. In this video we. Magnesium Bromide Water Solution.
From www.chegg.com
Solved A chemist prepares a solution of magnesium bromide Magnesium Bromide Water Solution See the references and methods. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. It is used as a sedative, a brominating agent, and. A magnesium cation (\mg^ {2+}\) and two bromide anions br− b r. Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. [3]. Magnesium Bromide Water Solution.
From www.indiamart.com
Liquid Methyl Magnesium Bromide, Packaging Type Drum, Packaging Size Magnesium Bromide Water Solution Find the standard enthalpy, entropy, and heat capacity of magnesium bromide in gas, liquid, and solid phases. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Magnesium + bromine → magnesium bromide. Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. [3] it can also be. See the references. Magnesium Bromide Water Solution.
From hxexfyicy.blob.core.windows.net
Magnesium Bromide And Sodium Sulfate at Sharon Jacques blog Magnesium Bromide Water Solution Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. [3] it can also be. Describe how an aqueous solution is formed from both ionic compounds. It is used as a sedative, a brominating agent, and. See the. Magnesium Bromide Water Solution.
From www.mcguff.com
Ipratropium Bromide, 0.02, Inhalation Solution, 2.5mL, 25 Vials/Tray Magnesium Bromide Water Solution Define a solution and describe the parts of a solution. It is used as a sedative, a brominating agent, and. [3] it can also be. A magnesium cation (\mg^ {2+}\) and two bromide anions br− b r. Describe how an aqueous solution is formed from both ionic compounds. Find the standard enthalpy, entropy, and heat capacity of magnesium bromide in. Magnesium Bromide Water Solution.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Magnesium Bromide Water Solution Describe how an aqueous solution is formed from both ionic compounds. Calcium carbonate → calcium oxide + carbon dioxide. In this video we will describe the equation mgbr2 + h2o and write what happens when mgbr2 is. [3] it can also be. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2.. Magnesium Bromide Water Solution.
From www.indiamart.com
VINYL MAGNESIUM BROMIDE SOLUTION at Rs 200/kg Ethylmagnesium Bromide Magnesium Bromide Water Solution It is used as a sedative, a brominating agent, and. [3] it can also be. Describe how an aqueous solution is formed from both ionic compounds. Magnesium bromide can be synthesized by treating magnesium oxide (and related basic salts) with hydrobromic acid. In this video we will describe the equation mgbr2 + h2o and write what happens when mgbr2 is.. Magnesium Bromide Water Solution.
From www.aluminummanufacturers.org
Aluminum Bromide Aluminum Sulfate Aluminum Manufacturers Magnesium Bromide Water Solution See the references and methods. Find the standard enthalpy, entropy, and heat capacity of magnesium bromide in gas, liquid, and solid phases. Describe how an aqueous solution is formed from both ionic compounds. Magnesium bromide can be synthesized by treating magnesium oxide (and related basic salts) with hydrobromic acid. A magnesium cation (\mg^ {2+}\) and two bromide anions br− b. Magnesium Bromide Water Solution.
From www.drsebiscellfood.com
Bromide Plus Powder Dr. Sebi's Cell Food Magnesium Bromide Water Solution In this video we will describe the equation mgbr2 + h2o and write what happens when mgbr2 is. Magnesium + bromine → magnesium bromide. Calcium carbonate → calcium oxide + carbon dioxide. [3] it can also be. Describe how an aqueous solution is formed from both ionic compounds. Magnesium bromide can be synthesized by treating magnesium oxide (and related basic. Magnesium Bromide Water Solution.
From chemcraft.su
Lead(II) bromide, 99.5 pure chemcraft.su Magnesium Bromide Water Solution A magnesium cation (\mg^ {2+}\) and two bromide anions br− b r. It is used as a sedative, a brominating agent, and. Magnesium bromide can be synthesized by treating magnesium oxide (and related basic salts) with hydrobromic acid. Describe how an aqueous solution is formed from both ionic compounds. Magnesium bromide is a white crystalline salt that dissolves in water. Magnesium Bromide Water Solution.
From www.solcohealthcare.com
Rocuronium Bromide Injection Solco Healthcare Magnesium Bromide Water Solution Calcium carbonate → calcium oxide + carbon dioxide. Describe how an aqueous solution is formed from both ionic compounds. See the references and methods. Find the standard enthalpy, entropy, and heat capacity of magnesium bromide in gas, liquid, and solid phases. Magnesium + bromine → magnesium bromide. A magnesium cation (\mg^ {2+}\) and two bromide anions br− b r. Define. Magnesium Bromide Water Solution.
From www.numerade.com
The electrolysis of an aqueous solution of magnesium bromide (MgBr2 Magnesium Bromide Water Solution Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. Calcium carbonate → calcium oxide + carbon dioxide. In this video we will describe the equation mgbr2 + h2o and write what happens when mgbr2 is. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Magnesium bromide is a white. Magnesium Bromide Water Solution.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Magnesium Bromide Water Solution Magnesium bromide can be synthesized by treating magnesium oxide (and related basic salts) with hydrobromic acid. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. See the references and methods. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. In this video we will describe. Magnesium Bromide Water Solution.
From www.indiamart.com
BioTech Grade Powder Sodium Bromide for Petroleum Industry, for Magnesium Bromide Water Solution Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Describe how an aqueous solution is formed from both ionic compounds. It is used as a sedative, a brominating agent, and. In this video we will describe the equation mgbr2 + h2o and write what happens when mgbr2 is. Magnesium bromide is a white crystalline. Magnesium Bromide Water Solution.
From www.thistlescientific.co.uk
Ethidium Bromide solution 10mg/ml Thistle Scientific Magnesium Bromide Water Solution Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. In this video we will describe the equation mgbr2 + h2o and write what happens when mgbr2 is. Calcium carbonate → calcium oxide + carbon dioxide. See the references and methods. Describe how an aqueous solution is formed from both ionic compounds. Magnesium bromide is. Magnesium Bromide Water Solution.
From www.sciencecompany.com
Reagent grade Potassium Bromide, 500g for sale from The Science Company. Magnesium Bromide Water Solution Find the standard enthalpy, entropy, and heat capacity of magnesium bromide in gas, liquid, and solid phases. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. [3] it can also be. In this video we will describe the equation mgbr2 + h2o and write what happens when mgbr2 is. Magnesium bromide. Magnesium Bromide Water Solution.
From www.sigmaaldrich.co.th
THIAZOLYL BLUE TETRAZOLIU M BROMIDE, 98 Merck Life Sciences Thailand Magnesium Bromide Water Solution It is used as a sedative, a brominating agent, and. Find the standard enthalpy, entropy, and heat capacity of magnesium bromide in gas, liquid, and solid phases. Describe how an aqueous solution is formed from both ionic compounds. Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. Learn about and revise electrolysis with this bbc. Magnesium Bromide Water Solution.
From www.sigmaaldrich.co.th
PYRIDINIUM BROMIDE PERBROMIDE, TECH., 90 Merck Life Sciences Thailand Magnesium Bromide Water Solution See the references and methods. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. It is used as a sedative, a brominating agent, and. Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. Calcium carbonate → calcium oxide + carbon dioxide. Magnesium + bromine → magnesium bromide. Find the. Magnesium Bromide Water Solution.
From www.grainger.com
7758023, 119, Potassium Bromide, Crystal, Reagent, ACS 6MMU0P1220 Magnesium Bromide Water Solution Magnesium + bromine → magnesium bromide. Magnesium bromide can be synthesized by treating magnesium oxide (and related basic salts) with hydrobromic acid. Find the standard enthalpy, entropy, and heat capacity of magnesium bromide in gas, liquid, and solid phases. In this video we will describe the equation mgbr2 + h2o and write what happens when mgbr2 is. Describe how an. Magnesium Bromide Water Solution.
From www.bhphotovideo.com
Photographers' Formulary Sodium Bromide 1 Lb. 101180 1LB B&H Magnesium Bromide Water Solution Calcium carbonate → calcium oxide + carbon dioxide. See the references and methods. Find the standard enthalpy, entropy, and heat capacity of magnesium bromide in gas, liquid, and solid phases. [3] it can also be. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. A magnesium cation (\mg^ {2+}\) and two. Magnesium Bromide Water Solution.
From www.grainger.com
57090, 364.48, Cetyltrimethylammonium Bromide 6PLA0CE122500GM Magnesium Bromide Water Solution Describe how an aqueous solution is formed from both ionic compounds. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Calcium carbonate → calcium oxide + carbon dioxide. Magnesium bromide can be synthesized by treating magnesium oxide (and related basic salts) with hydrobromic acid. [3] it can also be. A magnesium cation (\mg^ {2+}\). Magnesium Bromide Water Solution.
From www.fishersci.co.uk
Bromoacetyl bromide, 98, Thermo Scientific Chemicals Fisher Scientific Magnesium Bromide Water Solution Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. [3] it can also be. Magnesium bromide can be synthesized by treating magnesium oxide (and related basic salts) with hydrobromic acid. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Magnesium bromide is a white crystalline ionic compound with a. Magnesium Bromide Water Solution.
From www.macsenlab.com
Pinaverium Bromide API 53251948 Manufacturer & Supplier Magnesium Bromide Water Solution Describe how an aqueous solution is formed from both ionic compounds. Define a solution and describe the parts of a solution. Find the standard enthalpy, entropy, and heat capacity of magnesium bromide in gas, liquid, and solid phases. It is used as a sedative, a brominating agent, and. Calcium carbonate → calcium oxide + carbon dioxide. [3] it can also. Magnesium Bromide Water Solution.
From www.rpicorp.com
E718005.0 Ethidium Bromide, Powder, 5 Grams Magnesium Bromide Water Solution Define a solution and describe the parts of a solution. It is used as a sedative, a brominating agent, and. Magnesium + bromine → magnesium bromide. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. Magnesium bromide can be synthesized by treating magnesium oxide (and related basic salts) with hydrobromic acid.. Magnesium Bromide Water Solution.
From www.chemkits.eu
Potassium bromide, 98.5, 7758023 Magnesium Bromide Water Solution Define a solution and describe the parts of a solution. Calcium carbonate → calcium oxide + carbon dioxide. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Magnesium bromide is a white crystalline salt that dissolves in water and conducts electricity. Describe how an aqueous solution is formed from both ionic compounds. In this. Magnesium Bromide Water Solution.