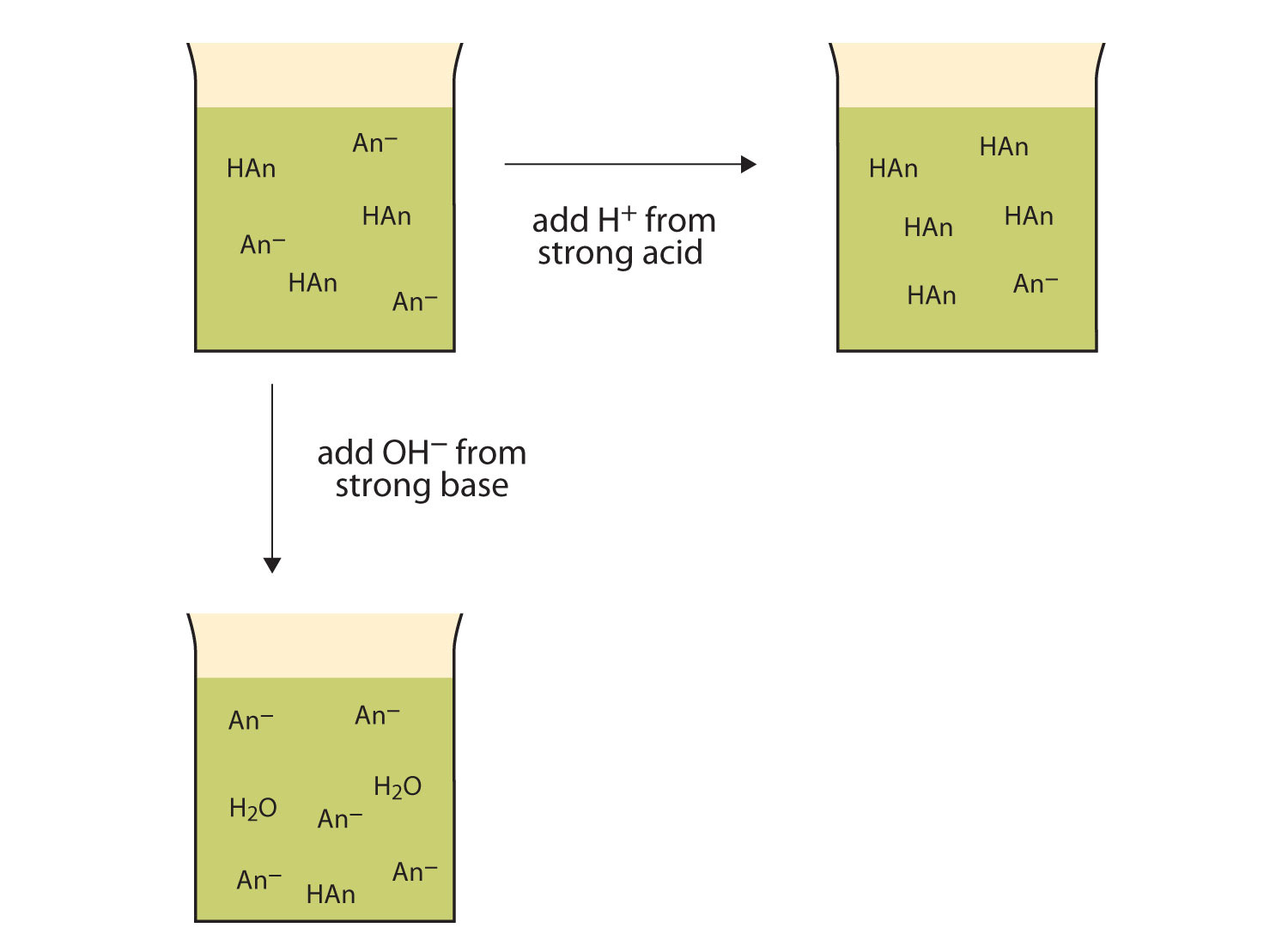
Mechanism Of Buffer Action Of Acidic Buffer . The mechanism of buffer action can be understood by considering an acidic buffer made of a weak acid like acetic acid and its sodium salt sodium acetate. To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). Ch 3 coona (aq) 3 Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. Mechanism of action of an acidic buffer : To understand the mechanism of buffer action, we can take. (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. An example of a buffer. How do buffer solutions work? A solution of acetic acid (ch3cooh ch 3 cooh and sodium acetate ch3coona ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. In the aqueous medium, they dissociate as:
from saylordotorg.github.io
To understand the mechanism of buffer action, we can take. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). How do buffer solutions work? The mechanism of buffer action can be understood by considering an acidic buffer made of a weak acid like acetic acid and its sodium salt sodium acetate. A solution of acetic acid (ch3cooh ch 3 cooh and sodium acetate ch3coona ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. In the aqueous medium, they dissociate as: Mechanism of action of an acidic buffer : (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. An example of a buffer.
Buffers
Mechanism Of Buffer Action Of Acidic Buffer A solution of acetic acid (ch3cooh ch 3 cooh and sodium acetate ch3coona ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. An example of a buffer. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. Ch 3 coona (aq) 3 How do buffer solutions work? To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. In the aqueous medium, they dissociate as: To understand the mechanism of buffer action, we can take. The mechanism of buffer action can be understood by considering an acidic buffer made of a weak acid like acetic acid and its sodium salt sodium acetate. A solution of acetic acid (ch3cooh ch 3 cooh and sodium acetate ch3coona ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. Mechanism of action of an acidic buffer :
From www.youtube.com
Difference between Acidic Buffer and Basic Buffer Buffer Types Mechanism Of Buffer Action Of Acidic Buffer Ch 3 coona (aq) 3 A solution of acetic acid (ch3cooh ch 3 cooh and sodium acetate ch3coona ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. How do buffer solutions work? The. Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideserve.com
PPT Mechanism of buffer action and buffer preparation PowerPoint Mechanism Of Buffer Action Of Acidic Buffer Ch 3 coona (aq) 3 To understand the mechanism of buffer action, we can take. Mechanism of action of an acidic buffer : (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. In the aqueous medium, they dissociate as: To understand the mechanism of buffer action, let us take an example. Mechanism Of Buffer Action Of Acidic Buffer.
From slidetodoc.com
Mechanism of buffer action and buffer preparation Maintaining Mechanism Of Buffer Action Of Acidic Buffer Mechanism of action of an acidic buffer : To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). To understand the mechanism of buffer action, we can take. An example of a buffer. In the aqueous medium, they dissociate as: A. Mechanism Of Buffer Action Of Acidic Buffer.
From www.doubtnut.com
[Marathi] Explain the mechanism of buffer action of an acidic buffer. Mechanism Of Buffer Action Of Acidic Buffer (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. Ch 3 coona (aq) 3 To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). Mechanism of action of an acidic buffer :. Mechanism Of Buffer Action Of Acidic Buffer.
From www.youtube.com
Buffer action in the blood YouTube Mechanism Of Buffer Action Of Acidic Buffer Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. How do buffer solutions work? In the aqueous medium, they dissociate as: To understand the mechanism. Mechanism Of Buffer Action Of Acidic Buffer.
From present5.com
BUFFER SOLUTIONS Buffer solutions solution which can resist Mechanism Of Buffer Action Of Acidic Buffer Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. In the aqueous medium, they dissociate as: The mechanism of buffer action can be understood by considering an acidic buffer made of a weak acid like acetic acid and its sodium salt sodium acetate.. Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Mechanism Of Buffer Action Of Acidic Buffer To understand the mechanism of buffer action, we can take. Ch 3 coona (aq) 3 To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). A solution of acetic acid (ch3cooh ch 3 cooh and sodium acetate ch3coona ch 3 coona). Mechanism Of Buffer Action Of Acidic Buffer.
From www.youtube.com
Buffer Action Acidic and basic buffer Action how buffer resist pH Mechanism Of Buffer Action Of Acidic Buffer An example of a buffer. To understand the mechanism of buffer action, we can take. Mechanism of action of an acidic buffer : Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. (1) an acidic buffer is a mixture of a weak acid. Mechanism Of Buffer Action Of Acidic Buffer.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Mechanism Of Buffer Action Of Acidic Buffer Ch 3 coona (aq) 3 To understand the mechanism of buffer action, we can take. The mechanism of buffer action can be understood by considering an acidic buffer made of a weak acid like acetic acid and its sodium salt sodium acetate. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes. Mechanism Of Buffer Action Of Acidic Buffer.
From www.youtube.com
Buffer Solution Buffer Action Types of buffer solution acidic and Mechanism Of Buffer Action Of Acidic Buffer (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. Ch 3 coona (aq) 3 The mechanism of buffer action can be understood by considering an acidic buffer made of a weak acid like acetic acid and its sodium salt sodium acetate. An example of a buffer. How do buffer solutions work?. Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideserve.com
PPT Mechanism of buffer action and buffer preparation PowerPoint Mechanism Of Buffer Action Of Acidic Buffer (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. A solution of acetic acid (ch3cooh ch 3 cooh and sodium acetate ch3coona ch 3 coona). Mechanism Of Buffer Action Of Acidic Buffer.
From www.youtube.com
buffer actions electrochemistry class 12 chemistry subject notes Mechanism Of Buffer Action Of Acidic Buffer In the aqueous medium, they dissociate as: The mechanism of buffer action can be understood by considering an acidic buffer made of a weak acid like acetic acid and its sodium salt sodium acetate. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added.. Mechanism Of Buffer Action Of Acidic Buffer.
From studyx.ai
1 What is a buffer solution Explain mechanism StudyX Mechanism Of Buffer Action Of Acidic Buffer The mechanism of buffer action can be understood by considering an acidic buffer made of a weak acid like acetic acid and its sodium salt sodium acetate. Mechanism of action of an acidic buffer : A solution of acetic acid (ch3cooh ch 3 cooh and sodium acetate ch3coona ch 3 coona) is an example of a buffer that consists of. Mechanism Of Buffer Action Of Acidic Buffer.
From saylordotorg.github.io
Buffers Mechanism Of Buffer Action Of Acidic Buffer Ch 3 coona (aq) 3 A solution of acetic acid (ch3cooh ch 3 cooh and sodium acetate ch3coona ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. The mechanism of buffer action can be understood by considering an acidic buffer made of a weak acid like acetic acid and its sodium. Mechanism Of Buffer Action Of Acidic Buffer.
From www.youtube.com
Mechanism of Acidic Buffer and Basic Buffer Solution Chemical Mechanism Of Buffer Action Of Acidic Buffer The mechanism of buffer action can be understood by considering an acidic buffer made of a weak acid like acetic acid and its sodium salt sodium acetate. Mechanism of action of an acidic buffer : To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium. Mechanism Of Buffer Action Of Acidic Buffer.
From slidetodoc.com
Mechanism of buffer action and buffer preparation Maintaining Mechanism Of Buffer Action Of Acidic Buffer Ch 3 coona (aq) 3 (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. An example of a buffer. To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). The mechanism of. Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideserve.com
PPT Mechanism of buffer action and buffer preparation PowerPoint Mechanism Of Buffer Action Of Acidic Buffer A solution of acetic acid (ch3cooh ch 3 cooh and sodium acetate ch3coona ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. Mechanism of action. Mechanism Of Buffer Action Of Acidic Buffer.
From www.pinterest.co.kr
Mechanism of Buffer action of an acid buffer Buffer solution Mechanism Of Buffer Action Of Acidic Buffer Ch 3 coona (aq) 3 A solution of acetic acid (ch3cooh ch 3 cooh and sodium acetate ch3coona ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. In the aqueous medium, they dissociate as: The mechanism of buffer action can be understood by considering an acidic buffer made of a weak. Mechanism Of Buffer Action Of Acidic Buffer.
From www.toppr.com
What is buffer solution Explain the buffer action of acidic buffer Mechanism Of Buffer Action Of Acidic Buffer Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. The mechanism of buffer action can be understood by considering an acidic buffer made of a weak acid like acetic acid and its sodium salt sodium acetate. Ch 3 coona (aq) 3 To understand. Mechanism Of Buffer Action Of Acidic Buffer.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at Mechanism Of Buffer Action Of Acidic Buffer To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). To understand the mechanism of buffer action, we can take. Mechanism of action of an acidic buffer : An example of a buffer. How do buffer solutions work? A solution of. Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID7055849 Mechanism Of Buffer Action Of Acidic Buffer (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). Ch 3 coona (aq) 3 The mechanism of buffer action can be understood. Mechanism Of Buffer Action Of Acidic Buffer.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Mechanism Of Buffer Action Of Acidic Buffer Mechanism of action of an acidic buffer : (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. A solution of acetic acid (ch3cooh ch 3 cooh and sodium acetate ch3coona ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. To understand the. Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Mechanism Of Buffer Action Of Acidic Buffer To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. How do buffer solutions work?. Mechanism Of Buffer Action Of Acidic Buffer.
From slidetodoc.com
Mechanism of buffer action and buffer preparation Maintaining Mechanism Of Buffer Action Of Acidic Buffer Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). (1) an acidic buffer is. Mechanism Of Buffer Action Of Acidic Buffer.
From relationshipbetween.com
Difference Between Buffer Action And Buffer Capacity Relationship Between Mechanism Of Buffer Action Of Acidic Buffer Ch 3 coona (aq) 3 How do buffer solutions work? A solution of acetic acid (ch3cooh ch 3 cooh and sodium acetate ch3coona ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. Mechanism of action of an acidic buffer : The mechanism of buffer action can be understood by considering an. Mechanism Of Buffer Action Of Acidic Buffer.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Mechanism Of Buffer Action Of Acidic Buffer In the aqueous medium, they dissociate as: A solution of acetic acid (ch3cooh ch 3 cooh and sodium acetate ch3coona ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids. Mechanism Of Buffer Action Of Acidic Buffer.
From present5.com
BUFFER SOLUTIONS Buffer solutions solution which can resist Mechanism Of Buffer Action Of Acidic Buffer How do buffer solutions work? An example of a buffer. The mechanism of buffer action can be understood by considering an acidic buffer made of a weak acid like acetic acid and its sodium salt sodium acetate. To understand the mechanism of buffer action, we can take. Ch 3 coona (aq) 3 In the aqueous medium, they dissociate as: (1). Mechanism Of Buffer Action Of Acidic Buffer.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks Mechanism Of Buffer Action Of Acidic Buffer A solution of acetic acid (ch3cooh ch 3 cooh and sodium acetate ch3coona ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. Ch 3 coona (aq) 3 An example of a buffer. Mechanism. Mechanism Of Buffer Action Of Acidic Buffer.
From pharmacydocs.blogspot.com
Essential Pharma Documents 1205 Buffer Capacity Mechanism Of Buffer Action Of Acidic Buffer To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). Mechanism of action of an acidic buffer : Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or. Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideserve.com
PPT Mechanism of buffer action and buffer preparation PowerPoint Mechanism Of Buffer Action Of Acidic Buffer To understand the mechanism of buffer action, we can take. The mechanism of buffer action can be understood by considering an acidic buffer made of a weak acid like acetic acid and its sodium salt sodium acetate. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or. Mechanism Of Buffer Action Of Acidic Buffer.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs Mechanism Of Buffer Action Of Acidic Buffer Mechanism of action of an acidic buffer : In the aqueous medium, they dissociate as: Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. How do buffer solutions work? An example of a buffer. The mechanism of buffer action can be understood by. Mechanism Of Buffer Action Of Acidic Buffer.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Mechanism Of Buffer Action Of Acidic Buffer Ch 3 coona (aq) 3 (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. An example of a buffer. To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). A solution of. Mechanism Of Buffer Action Of Acidic Buffer.
From present5.com
BUFFER SOLUTIONS Buffer solutions solution which can resist Mechanism Of Buffer Action Of Acidic Buffer The mechanism of buffer action can be understood by considering an acidic buffer made of a weak acid like acetic acid and its sodium salt sodium acetate. To understand the mechanism of buffer action, we can take. (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. Ch 3 coona (aq) 3. Mechanism Of Buffer Action Of Acidic Buffer.
From slidetodoc.com
Mechanism of buffer action and buffer preparation Maintaining Mechanism Of Buffer Action Of Acidic Buffer How do buffer solutions work? (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. Mechanism of action of an acidic buffer : To understand the mechanism of buffer action, we can take. In the aqueous medium, they dissociate as: Ch 3 coona (aq) 3 Buffer action refers to the property of. Mechanism Of Buffer Action Of Acidic Buffer.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Mechanism Of Buffer Action Of Acidic Buffer How do buffer solutions work? An example of a buffer. Mechanism of action of an acidic buffer : In the aqueous medium, they dissociate as: To understand the mechanism of buffer action, we can take. (1) an acidic buffer is a mixture of a weak acid and its salt with a strong base. The mechanism of buffer action can be. Mechanism Of Buffer Action Of Acidic Buffer.