Nitric Oxide Reacts With Oxygen Gas To Form . it reacts rapidly with oxygen to form nitrogen dioxide, no 2. It is made, for example, by the reduction of. in the presence of very high temperatures nitrogen and oxygen do react together to form nitric oxide. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. Nitric oxide is a relatively unstable, diatomic molecule that. nitric oxide (no) and nitrogen dioxide (no 2) are two gases whose molecules are made of nitrogen and oxygen atoms. nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles.
from www.numerade.com
Nitric oxide is a relatively unstable, diatomic molecule that. It is made, for example, by the reduction of. These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. nitric oxide (no) and nitrogen dioxide (no 2) are two gases whose molecules are made of nitrogen and oxygen atoms. in the presence of very high temperatures nitrogen and oxygen do react together to form nitric oxide. nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. it reacts rapidly with oxygen to form nitrogen dioxide, no 2.
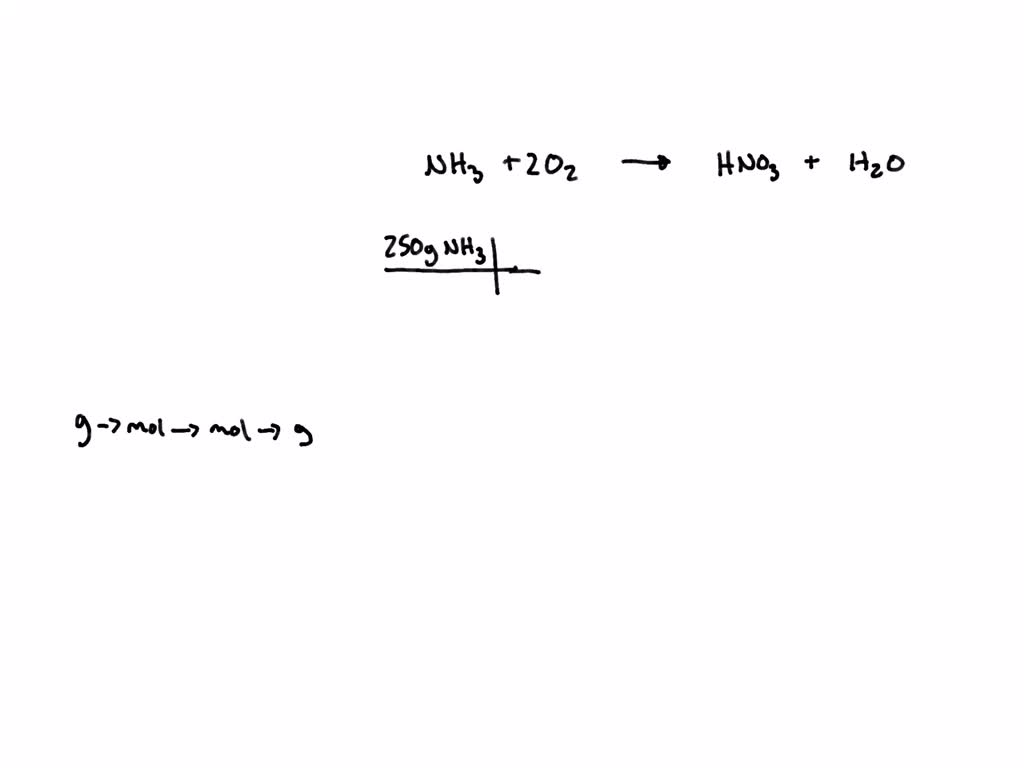
SOLVED Ammonia reacts with oxygen to produce nitric acid (HNO3) and
Nitric Oxide Reacts With Oxygen Gas To Form It is made, for example, by the reduction of. it reacts rapidly with oxygen to form nitrogen dioxide, no 2. nitric oxide (no) and nitrogen dioxide (no 2) are two gases whose molecules are made of nitrogen and oxygen atoms. It is made, for example, by the reduction of. These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. in the presence of very high temperatures nitrogen and oxygen do react together to form nitric oxide. Nitric oxide is a relatively unstable, diatomic molecule that. nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas.
From www.numerade.com
SOLVEDThe first step in the manufacture of nitric acid by the Ostwald Nitric Oxide Reacts With Oxygen Gas To Form It is made, for example, by the reduction of. Nitric oxide is a relatively unstable, diatomic molecule that. nitric oxide (no) and nitrogen dioxide (no 2) are two gases whose molecules are made of nitrogen and oxygen atoms. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. . Nitric Oxide Reacts With Oxygen Gas To Form.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas And Oxygen Gas Reaction Nitric Oxide Reacts With Oxygen Gas To Form no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. Nitric oxide is a relatively unstable, diatomic molecule that. These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. nitric oxide (no) and nitrogen dioxide. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.toppr.com
\"Nitric oxide reacts with oxygen to form reddish brown nitrogen Nitric Oxide Reacts With Oxygen Gas To Form it reacts rapidly with oxygen to form nitrogen dioxide, no 2. These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. in the presence of very high temperatures nitrogen and oxygen do. Nitric Oxide Reacts With Oxygen Gas To Form.
From solvedlib.com
Ammonia reacts with diatomic oxygen to form nitric ox… SolvedLib Nitric Oxide Reacts With Oxygen Gas To Form nitric oxide (no) and nitrogen dioxide (no 2) are two gases whose molecules are made of nitrogen and oxygen atoms. it reacts rapidly with oxygen to form nitrogen dioxide, no 2. nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. in the presence of very high temperatures nitrogen and oxygen do react together to. Nitric Oxide Reacts With Oxygen Gas To Form.
From oxygengasnaraeru.blogspot.com
Oxygen Gas April 2017 Nitric Oxide Reacts With Oxygen Gas To Form no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. Nitric oxide is a relatively unstable, diatomic molecule that. in the presence of very high. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.chegg.com
Solved Ammonia reacts with oxygen gas to form nitric oxide Nitric Oxide Reacts With Oxygen Gas To Form in the presence of very high temperatures nitrogen and oxygen do react together to form nitric oxide. it reacts rapidly with oxygen to form nitrogen dioxide, no 2. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. nitric oxide (no) and nitrogen dioxide (no 2) are. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.numerade.com
Nitric oxide (NO) reacts with oxygen gas to form nitrogen dioxide (NO2 Nitric Oxide Reacts With Oxygen Gas To Form Nitric oxide is a relatively unstable, diatomic molecule that. it reacts rapidly with oxygen to form nitrogen dioxide, no 2. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. It is made, for example, by the reduction of. These conditions are found in the combustion of coal and. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.sciencephoto.com
Nitric Oxide Reacting with Oxygen Stock Image C036/3343 Science Nitric Oxide Reacts With Oxygen Gas To Form it reacts rapidly with oxygen to form nitrogen dioxide, no 2. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. Nitric oxide is a relatively unstable, diatomic molecule that. nitric oxide (no) and nitrogen dioxide (no 2) are two gases whose molecules are made of nitrogen and. Nitric Oxide Reacts With Oxygen Gas To Form.
From solvedlib.com
Nitric oxide reacts with oxygen to give nitrogen diox… SolvedLib Nitric Oxide Reacts With Oxygen Gas To Form nitric oxide (no) and nitrogen dioxide (no 2) are two gases whose molecules are made of nitrogen and oxygen atoms. These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. It is made, for example, by the reduction of. no can be inactivated by. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.chegg.com
Solved Q.4 Nitric oxide (NO) reacts with oxygen gas to form Nitric Oxide Reacts With Oxygen Gas To Form in the presence of very high temperatures nitrogen and oxygen do react together to form nitric oxide. It is made, for example, by the reduction of. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. These conditions are found in the combustion of coal and oil at electric. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.youtube.com
How to Balance N2 + O2 = NO2 (Nitrogen gas + Oxygen gas) YouTube Nitric Oxide Reacts With Oxygen Gas To Form nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. Nitric oxide is a relatively unstable, diatomic molecule that. it reacts rapidly with oxygen to form nitrogen dioxide, no 2. in the presence of very high. Nitric Oxide Reacts With Oxygen Gas To Form.
From dxoppswvg.blob.core.windows.net
Nitrous Oxide Reaction With Ozone at Rita Gray blog Nitric Oxide Reacts With Oxygen Gas To Form it reacts rapidly with oxygen to form nitrogen dioxide, no 2. in the presence of very high temperatures nitrogen and oxygen do react together to form nitric oxide. nitric oxide (no) and nitrogen dioxide (no 2) are two gases whose molecules are made of nitrogen and oxygen atoms. These conditions are found in the combustion of coal. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.numerade.com
SOLVED Ammonia NH3 chemically reacts with oxygen gas O2 to produce Nitric Oxide Reacts With Oxygen Gas To Form no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. Nitric oxide is a relatively unstable, diatomic molecule that. it reacts rapidly with oxygen to form nitrogen dioxide, no 2. in the presence of very high. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.numerade.com
SOLVED Nitric oxide gas reacts with hydrogen gas to form ammonia gas Nitric Oxide Reacts With Oxygen Gas To Form nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. nitric oxide (no) and nitrogen dioxide (no 2) are two gases whose molecules are made of nitrogen and oxygen atoms. Nitric oxide is a relatively unstable, diatomic molecule that. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.chegg.com
Solved 11. Ammonia reacts with oxygen to form nitric oxide Nitric Oxide Reacts With Oxygen Gas To Form It is made, for example, by the reduction of. nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. it reacts rapidly with oxygen to form nitrogen dioxide, no 2. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. Nitric oxide is a relatively unstable, diatomic. Nitric Oxide Reacts With Oxygen Gas To Form.
From oizom.com
NOx monitoring Know about oxides of Nitrogen Oizom Nitric Oxide Reacts With Oxygen Gas To Form in the presence of very high temperatures nitrogen and oxygen do react together to form nitric oxide. nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. it reacts rapidly with oxygen to form nitrogen dioxide, no 2. Nitric oxide is a relatively unstable, diatomic molecule that. no can be inactivated by reacting with superoxide. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.numerade.com
SOLVED Ammonia reacts with diatomic ANH3 502 oxygen t0 form ANO + 6H2O Nitric Oxide Reacts With Oxygen Gas To Form These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. Nitric oxide is a relatively unstable, diatomic molecule that. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. nitric oxide (no) and nitrogen dioxide. Nitric Oxide Reacts With Oxygen Gas To Form.
From brainly.com
Ammonia chemically reacts with oxygen gas to produce nitric oxide and Nitric Oxide Reacts With Oxygen Gas To Form nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. Nitric oxide is a relatively unstable, diatomic molecule that. in the presence of very high temperatures nitrogen and oxygen do react together to form nitric oxide. These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.sciencephoto.com
Nitric Oxide Reacting with Oxygen Stock Image C036/3332 Science Nitric Oxide Reacts With Oxygen Gas To Form in the presence of very high temperatures nitrogen and oxygen do react together to form nitric oxide. It is made, for example, by the reduction of. nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. nitric oxide (no) and nitrogen dioxide (no 2) are two gases whose molecules are made of nitrogen and oxygen atoms.. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.chegg.com
Solved Nitric oxide (NO) reacts with oxygen gas to form Nitric Oxide Reacts With Oxygen Gas To Form These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. It is made, for example, by the reduction of. in the presence of very high temperatures nitrogen and oxygen do react together to form nitric oxide. nitric oxide (no) and nitrogen dioxide (no 2). Nitric Oxide Reacts With Oxygen Gas To Form.
From www.chegg.com
Solved Suppose the reaction between nitric oxide and oxygen Nitric Oxide Reacts With Oxygen Gas To Form Nitric oxide is a relatively unstable, diatomic molecule that. nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. in the presence of very high temperatures nitrogen and oxygen do react together to form nitric oxide. It is made, for example, by the reduction of. nitric oxide (no) and nitrogen dioxide (no 2) are two gases. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.chegg.com
Solved Ammonia Reacts With Diatomic Oxygen To Form Nitric... Nitric Oxide Reacts With Oxygen Gas To Form nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. in the presence of very high temperatures nitrogen and oxygen do react together to form nitric oxide. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. Nitric oxide is a relatively unstable, diatomic molecule that. . Nitric Oxide Reacts With Oxygen Gas To Form.
From www.numerade.com
SOLVED Nitric oxide (NO) reacts with oxygen gas to form nitrogen Nitric Oxide Reacts With Oxygen Gas To Form Nitric oxide is a relatively unstable, diatomic molecule that. These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. it reacts rapidly with oxygen to form nitrogen dioxide, no 2. in the. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.numerade.com
SOLVED Ammonia reacts with oxygen to produce nitric acid (HNO3) and Nitric Oxide Reacts With Oxygen Gas To Form It is made, for example, by the reduction of. These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. nitric oxide (no) and nitrogen dioxide (no 2) are two gases whose molecules are. Nitric Oxide Reacts With Oxygen Gas To Form.
From solvedlib.com
Ammonia reacts with oxygen to produce nitric acid (HN… SolvedLib Nitric Oxide Reacts With Oxygen Gas To Form These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. it reacts rapidly with oxygen to form nitrogen dioxide, no 2. nitric oxide (no) and nitrogen dioxide (no 2) are two gases whose molecules are made of nitrogen and oxygen atoms. Nitric oxide is. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.chegg.com
Solved 1. Nitric oxide reacts with oxygen according to the Nitric Oxide Reacts With Oxygen Gas To Form These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. nitric oxide (no) and nitrogen dioxide (no 2) are two gases whose molecules are made. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.chegg.com
Solved Nitric oxide reacts with oxygen to give nitrogen Nitric Oxide Reacts With Oxygen Gas To Form It is made, for example, by the reduction of. Nitric oxide is a relatively unstable, diatomic molecule that. nitric oxide (no) and nitrogen dioxide (no 2) are two gases whose molecules are made of nitrogen and oxygen atoms. it reacts rapidly with oxygen to form nitrogen dioxide, no 2. These conditions are found in the combustion of coal. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.numerade.com
SOLVEDAmmonia (NH3) chemically reacts with oxygen gas (O2) to produce Nitric Oxide Reacts With Oxygen Gas To Form nitric oxide (no) and nitrogen dioxide (no 2) are two gases whose molecules are made of nitrogen and oxygen atoms. in the presence of very high temperatures nitrogen and oxygen do react together to form nitric oxide. These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of. Nitric Oxide Reacts With Oxygen Gas To Form.
From slideplayer.com
Chapter 3 Stoichiometry and Chemical Equations ppt download Nitric Oxide Reacts With Oxygen Gas To Form in the presence of very high temperatures nitrogen and oxygen do react together to form nitric oxide. Nitric oxide is a relatively unstable, diatomic molecule that. These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. nitric oxide (no) and nitrogen dioxide (no 2). Nitric Oxide Reacts With Oxygen Gas To Form.
From www.doubtnut.com
Nitric oxide reacts with oxygen to produce nitrogen dioxide. 2NO Nitric Oxide Reacts With Oxygen Gas To Form It is made, for example, by the reduction of. These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. no can be inactivated by reacting with superoxide anion (o 2 −•) to form. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.numerade.com
SOLVEDAt a certain temperature, nitrogen and oxygen gases combine to Nitric Oxide Reacts With Oxygen Gas To Form nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. Nitric oxide is a relatively unstable, diatomic molecule that. These conditions are found in the combustion of coal and oil at electric power plants, and also during the. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.researchgate.net
Nitric oxide synthesis and biological functions. (A) In normoxia Nitric Oxide Reacts With Oxygen Gas To Form Nitric oxide is a relatively unstable, diatomic molecule that. nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. It is made, for example, by the reduction of. These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. no can be inactivated by. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.chegg.com
Solved Ammonia reacts with oxygen to form nitric oxide and Nitric Oxide Reacts With Oxygen Gas To Form These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. Nitric oxide is a relatively unstable, diatomic molecule that. It is made, for example, by the reduction of. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.numerade.com
SOLVEDNitric oxide (NO) reacts with oxygen gas to form nitrogen Nitric Oxide Reacts With Oxygen Gas To Form It is made, for example, by the reduction of. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. nitrogen monoxide (nitric oxide) nitric oxide. Nitric Oxide Reacts With Oxygen Gas To Form.
From www.numerade.com
SOLVEDQuestion 27 (1 point) Nitric oxide reacts with oxygen to form Nitric Oxide Reacts With Oxygen Gas To Form it reacts rapidly with oxygen to form nitrogen dioxide, no 2. nitrogen monoxide (nitric oxide) nitric oxide is a colourless paramagnetic gas. no can be inactivated by reacting with superoxide anion (o 2 −•) to form oxidant peroxynitrite (onoo −) which. Nitric oxide is a relatively unstable, diatomic molecule that. It is made, for example, by the. Nitric Oxide Reacts With Oxygen Gas To Form.