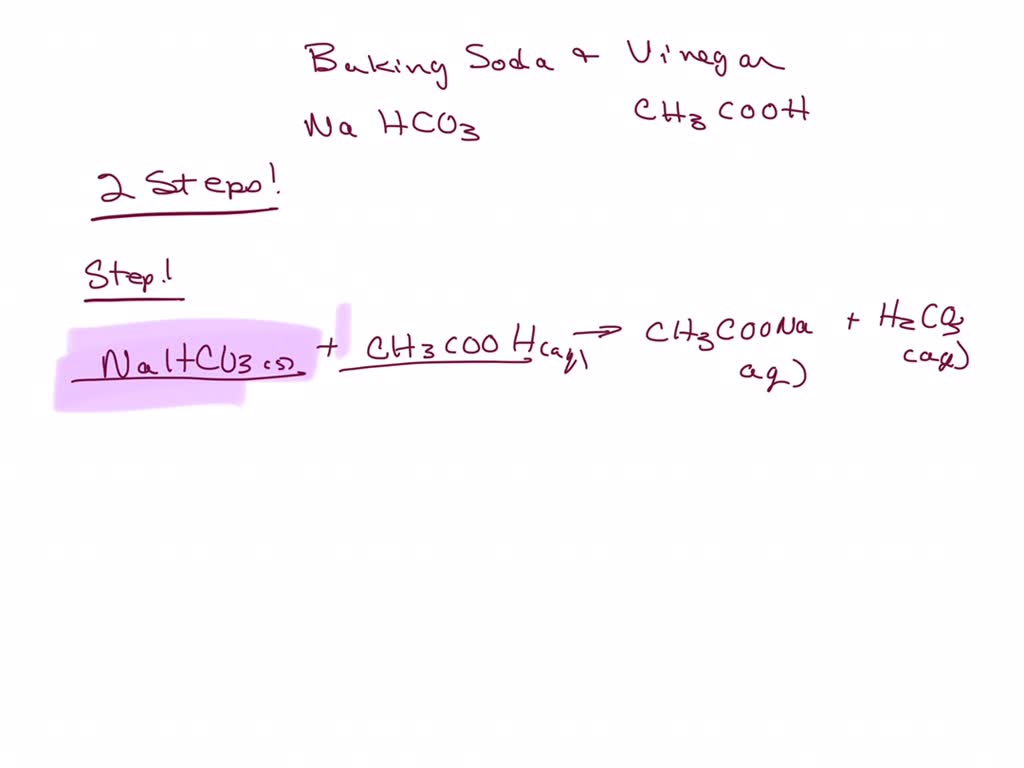
Baking Soda And Vinegar Equation . The second reaction is a decomposition reaction. Carbonic acid and sodium acetate. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). What happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: The combination of baking soda and vinegar is. The water in the vinegar acts as a host where the base and acid react. Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion. It explains how to write the net ionic. This chemistry video tutorial discusses the reaction between baking soda and vinegar. Baking soda is sodium bi carbonate. The reaction causes the baking soda to transform into water and carbon dioxide. The result of this initial reaction is two new chemicals: In chemistry, reactions are frequently written as an equation, using chemical symbols. Learn how baking soda and vinegar react to form carbon dioxide, water and ions. The reactants are on the left side of the equation,.
from www.numerade.com
This chemistry video tutorial discusses the reaction between baking soda and vinegar. The carbon dioxide is a gas which is released during the. The combination of baking soda and vinegar is. The water in the vinegar acts as a host where the base and acid react. The chemical equation for the overall reaction is: In chemistry, reactions are frequently written as an equation, using chemical symbols. Baking soda is sodium bi carbonate. Carbonic acid and sodium acetate. Explore fun science experiments with fizzy. Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion.
SOLVED The chemical equation for the reaction of baking soda (sodium
Baking Soda And Vinegar Equation The second reaction is a decomposition reaction. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. The result of this initial reaction is two new chemicals: The reactants are on the left side of the equation,. What happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: In chemistry, reactions are frequently written as an equation, using chemical symbols. It explains how to write the net ionic. Carbonic acid and sodium acetate. Explore fun science experiments with fizzy. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The chemical equation for the overall reaction is: This chemistry video tutorial discusses the reaction between baking soda and vinegar. The water in the vinegar acts as a host where the base and acid react. Learn how baking soda and vinegar react to form carbon dioxide, water and ions. The reaction causes the baking soda to transform into water and carbon dioxide. The combination of baking soda and vinegar is.
From sciencenotes.org
Chemical Equation for Baking Soda and Vinegar Reaction Baking Soda And Vinegar Equation In chemistry, reactions are frequently written as an equation, using chemical symbols. The chemical equation for the overall reaction is: The reactants are on the left side of the equation,. The second reaction is a decomposition reaction. Baking soda is sodium bi carbonate. It explains how to write the net ionic. Carbonic acid and sodium acetate. The water in the. Baking Soda And Vinegar Equation.
From chemistry291.blogspot.com
Baking Soda and Vinegar Chemical Reaction ExplanationNaHCO3 + CH3COOH Baking Soda And Vinegar Equation The second reaction is a decomposition reaction. This chemistry video tutorial discusses the reaction between baking soda and vinegar. The water in the vinegar acts as a host where the base and acid react. What happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: Baking soda. Baking Soda And Vinegar Equation.
From fyoilbwno.blob.core.windows.net
Baking Soda And Vinegar Formula Equation at Larry Padgett blog Baking Soda And Vinegar Equation The chemical equation for the overall reaction is: When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. The reactants are on the left side of the equation,. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes. Baking Soda And Vinegar Equation.
From asideload7.gitlab.io
Amazing Balanced Chemical Equation Of Vinegar And Baking Soda Baking Soda And Vinegar Equation This chemistry video tutorial discusses the reaction between baking soda and vinegar. Baking soda is sodium bi carbonate. Explore fun science experiments with fizzy. What happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: The water in the vinegar acts as a host where the base. Baking Soda And Vinegar Equation.
From www.vectorstock.com
Science experiment with baking soda and vinegar Vector Image Baking Soda And Vinegar Equation In chemistry, reactions are frequently written as an equation, using chemical symbols. Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion. The combination of baking soda and vinegar is. The second reaction is a decomposition reaction. Explore fun science experiments with fizzy. The reactants are on the left side of. Baking Soda And Vinegar Equation.
From stemmayhem.com
Why Do Vinegar & Baking Soda React? · STEM Mayhem Baking Soda And Vinegar Equation Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion. The second reaction is a decomposition reaction. The combination of baking soda and vinegar is. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). When vinegar and. Baking Soda And Vinegar Equation.
From www.pinterest.ca
A Simple Explanation the Difference Between Baking Soda & Baking Baking Soda And Vinegar Equation What happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: The chemical equation for the overall reaction is: The second reaction is a decomposition reaction. The water in the vinegar acts as a host where the base and acid react. The result of this initial reaction. Baking Soda And Vinegar Equation.
From exotjzliw.blob.core.windows.net
Vinegar + Baking Soda Chemical Equation at Allen Oneal blog Baking Soda And Vinegar Equation Baking soda is sodium bi carbonate. The chemical equation for the overall reaction is: During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). In chemistry, reactions are frequently written as an equation, using chemical symbols. The reactants are on the left side of the equation,. Explore. Baking Soda And Vinegar Equation.
From alfredoyouthweber.blogspot.com
Baking Soda and Vinegar Reaction Baking Soda And Vinegar Equation What happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: The combination of baking soda and vinegar is. Carbonic acid and sodium acetate. The reactants are on the left side of the equation,. Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water. Baking Soda And Vinegar Equation.
From www.slideserve.com
PPT Baking Soda/Vinegar Stoichiometry Lab PowerPoint Presentation Baking Soda And Vinegar Equation Learn how baking soda and vinegar react to form carbon dioxide, water and ions. The chemical equation for the overall reaction is: The combination of baking soda and vinegar is. Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion. During the reaction, when the baking soda is mixed with the. Baking Soda And Vinegar Equation.
From exotjzliw.blob.core.windows.net
Vinegar + Baking Soda Chemical Equation at Allen Oneal blog Baking Soda And Vinegar Equation Baking soda is sodium bi carbonate. The water in the vinegar acts as a host where the base and acid react. Explore fun science experiments with fizzy. What happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: The second reaction is a decomposition reaction. The reactants. Baking Soda And Vinegar Equation.
From www.numerade.com
SOLVED The chemical equation for the reaction of baking soda (sodium Baking Soda And Vinegar Equation Learn how baking soda and vinegar react to form carbon dioxide, water and ions. This chemistry video tutorial discusses the reaction between baking soda and vinegar. It explains how to write the net ionic. The reaction causes the baking soda to transform into water and carbon dioxide. The carbon dioxide is a gas which is released during the. The second. Baking Soda And Vinegar Equation.
From www.numerade.com
SOLVED Baking soda and vinegar react according to the following Baking Soda And Vinegar Equation This chemistry video tutorial discusses the reaction between baking soda and vinegar. The combination of baking soda and vinegar is. The result of this initial reaction is two new chemicals: Carbonic acid and sodium acetate. In chemistry, reactions are frequently written as an equation, using chemical symbols. The reaction causes the baking soda to transform into water and carbon dioxide.. Baking Soda And Vinegar Equation.
From www.youtube.com
Baking soda and vinegar reaction. ⚗️ 3 Experiments to do with kids ⚗️ Baking Soda And Vinegar Equation Explore fun science experiments with fizzy. The water in the vinegar acts as a host where the base and acid react. Baking soda is sodium bi carbonate. Learn how baking soda and vinegar react to form carbon dioxide, water and ions. The combination of baking soda and vinegar is. The reactants are on the left side of the equation,. The. Baking Soda And Vinegar Equation.
From gioaihlpd.blob.core.windows.net
Baking Soda And Vinegar After Reaction at Alyson Olsen blog Baking Soda And Vinegar Equation The chemical equation for the overall reaction is: Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion. It explains how to write the net ionic. The reactants are on the left side of the equation,. The result of this initial reaction is two new chemicals: The second reaction is a. Baking Soda And Vinegar Equation.
From shotprofessional22.gitlab.io
Supreme Baking Soda And Vinegar Word Equation C Formula Physics Baking Soda And Vinegar Equation The reactants are on the left side of the equation,. What happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: The combination of baking soda and vinegar is. The carbon dioxide is a gas which is released during the. During the reaction, when the baking soda. Baking Soda And Vinegar Equation.
From paten38f.blogspot.com
Baking Soda And Vinegar / Vinegar And Baking Soda Tinkerlab / Everyday Baking Soda And Vinegar Equation The water in the vinegar acts as a host where the base and acid react. Carbonic acid and sodium acetate. Baking soda is sodium bi carbonate. What happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: During the reaction, when the baking soda is mixed with. Baking Soda And Vinegar Equation.
From lifeovercs.com
Baking Soda and Vinegar Preschoolers Science Experiment Life Over C's Baking Soda And Vinegar Equation What happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: The reaction causes the baking soda to transform into water and carbon dioxide. Carbonic acid and sodium acetate. The combination of baking soda and vinegar is. The reactants are on the left side of the equation,.. Baking Soda And Vinegar Equation.
From team-cartwright.com
Fizzy Heart Science Experiment Team Cartwright Baking Soda And Vinegar Equation The reactants are on the left side of the equation,. Explore fun science experiments with fizzy. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. The carbon dioxide is a gas which is released during the. During the reaction, when the baking soda is. Baking Soda And Vinegar Equation.
From www.youtube.com
Type of Reaction for Baking Soda and Vinegar ( NaHCO3 + CH3COOH) YouTube Baking Soda And Vinegar Equation It explains how to write the net ionic. Explore fun science experiments with fizzy. The combination of baking soda and vinegar is. The result of this initial reaction is two new chemicals: Learn how baking soda and vinegar react to form carbon dioxide, water and ions. The reaction causes the baking soda to transform into water and carbon dioxide. In. Baking Soda And Vinegar Equation.
From www.tessshebaylo.com
Chemical Equation For Baking Soda Citric Acid And Water Tessshebaylo Baking Soda And Vinegar Equation The chemical equation for the overall reaction is: Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion. The combination of baking soda and vinegar is. The water in the vinegar acts as a host where the base and acid react. Carbonic acid and sodium acetate. The reaction causes the baking. Baking Soda And Vinegar Equation.
From www.youtube.com
Chemical Reaction Of Baking Soda And Vinegar (Sodium Bicarbonate And Baking Soda And Vinegar Equation Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The result of this initial reaction is two new chemicals: Baking soda is sodium bi carbonate. Learn how. Baking Soda And Vinegar Equation.
From exotjzliw.blob.core.windows.net
Vinegar + Baking Soda Chemical Equation at Allen Oneal blog Baking Soda And Vinegar Equation The carbon dioxide is a gas which is released during the. It explains how to write the net ionic. The water in the vinegar acts as a host where the base and acid react. Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion. Explore fun science experiments with fizzy. Baking. Baking Soda And Vinegar Equation.
From www.youtube.com
Chemical reaction mixing baking soda and vinegar YouTube Baking Soda And Vinegar Equation The chemical equation for the overall reaction is: Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). In chemistry, reactions are frequently written as an equation, using. Baking Soda And Vinegar Equation.
From www.coursehero.com
[Solved] 6. The equation for the reaction between baking soda and Baking Soda And Vinegar Equation This chemistry video tutorial discusses the reaction between baking soda and vinegar. The combination of baking soda and vinegar is. Carbonic acid and sodium acetate. Baking soda is sodium bi carbonate. Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion. When vinegar and baking soda are first mixed together, hydrogen. Baking Soda And Vinegar Equation.
From www.youtube.com
Baking Soda and Vinegar Chemical Reaction (EXPERIMENT EXPLAINED) YouTube Baking Soda And Vinegar Equation When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. This chemistry video tutorial discusses the reaction between baking soda and vinegar. The reactants are on the left side of the equation,. What happens when vinegar reacts with baking soda occurs in two steps, but. Baking Soda And Vinegar Equation.
From www.ingridscience.ca
Baking soda and vinegar ingridscience.ca Baking Soda And Vinegar Equation It explains how to write the net ionic. The reactants are on the left side of the equation,. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The reaction causes the baking soda to transform into water and carbon dioxide. The carbon dioxide is a gas. Baking Soda And Vinegar Equation.
From chemistry291.blogspot.com
Baking Soda and Vinegar Chemical Reaction ExplanationNaHCO3 + CH3COOH Baking Soda And Vinegar Equation During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The water in the vinegar acts as a host where the base and acid react. Explore fun science experiments with fizzy. Carbonic acid and sodium acetate. The chemical equation for the overall reaction is: Learn how baking. Baking Soda And Vinegar Equation.
From www.youtube.com
vinegar and baking soda reaction YouTube Baking Soda And Vinegar Equation The chemical equation for the overall reaction is: The result of this initial reaction is two new chemicals: The second reaction is a decomposition reaction. Explore fun science experiments with fizzy. It explains how to write the net ionic. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions. Baking Soda And Vinegar Equation.
From chemistry291.blogspot.com
Baking Soda and Vinegar Chemical Reaction ExplanationNaHCO3 + CH3COOH Baking Soda And Vinegar Equation Baking soda is sodium bi carbonate. The second reaction is a decomposition reaction. The reaction causes the baking soda to transform into water and carbon dioxide. Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion. In chemistry, reactions are frequently written as an equation, using chemical symbols. Learn how baking. Baking Soda And Vinegar Equation.
From readingandwritingprojectcom.web.fc2.com
baking soda and vinegar reaction equation Baking Soda And Vinegar Equation The reaction causes the baking soda to transform into water and carbon dioxide. Carbonic acid and sodium acetate. The reactants are on the left side of the equation,. Baking soda is sodium bi carbonate. The chemical equation for the overall reaction is: It explains how to write the net ionic. In chemistry, reactions are frequently written as an equation, using. Baking Soda And Vinegar Equation.
From signalticket9.pythonanywhere.com
Marvelous Vinegar Plus Baking Soda Chemical Equation Balanced For And Baking Soda And Vinegar Equation The result of this initial reaction is two new chemicals: The reaction causes the baking soda to transform into water and carbon dioxide. Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion. The reactants are on the left side of the equation,. During the reaction, when the baking soda is. Baking Soda And Vinegar Equation.
From colouremployer8.gitlab.io
Unique Chemical Equation Of Vinegar And Baking Soda Electrostatic Notes Baking Soda And Vinegar Equation What happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: It explains how to write the net ionic. Carbonic acid and sodium acetate. The second reaction is a decomposition reaction. Baking soda is sodium bi carbonate. During the reaction, when the baking soda is mixed with. Baking Soda And Vinegar Equation.
From www.thoughtco.com
Equation for Reaction Between Baking Soda and Vinegar Baking Soda And Vinegar Equation The carbon dioxide is a gas which is released during the. In chemistry, reactions are frequently written as an equation, using chemical symbols. The combination of baking soda and vinegar is. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). Learn how baking soda and vinegar. Baking Soda And Vinegar Equation.
From www.youtube.com
Baking Soda and Vinegar Equation and Reaction YouTube Baking Soda And Vinegar Equation It explains how to write the net ionic. In chemistry, reactions are frequently written as an equation, using chemical symbols. Carbonic acid and sodium acetate. The chemical equation for the overall reaction is: Explore fun science experiments with fizzy. What happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the. Baking Soda And Vinegar Equation.