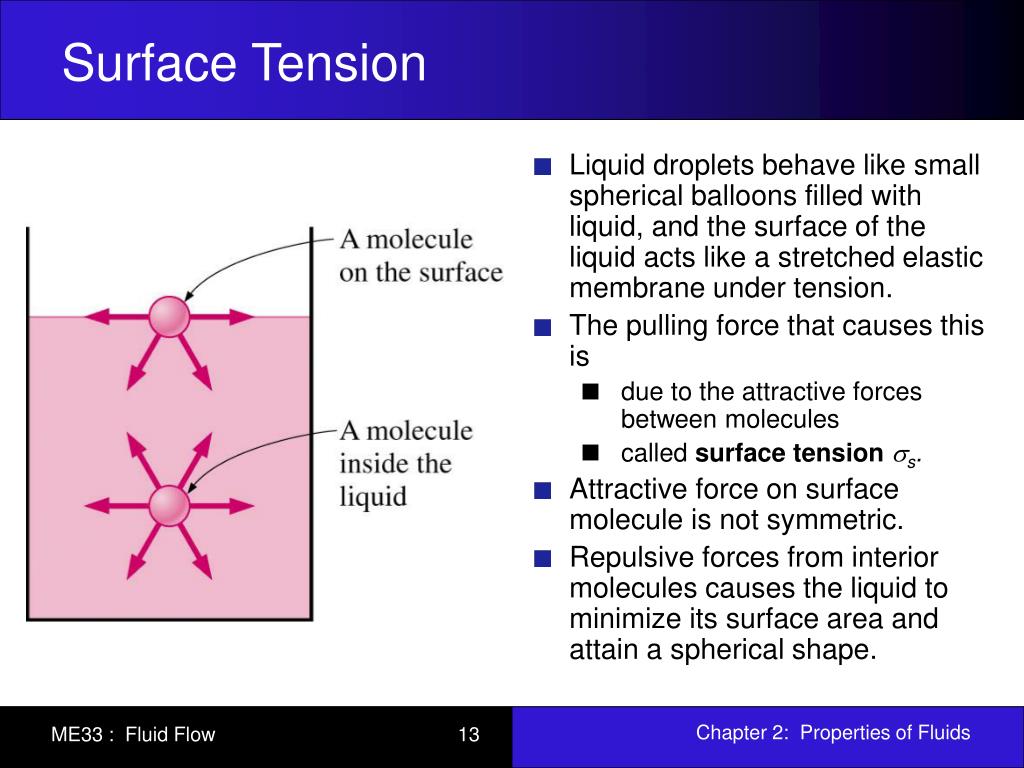
Surface Tension Worked Examples . An example is mercury, whose surface tension at 25 °c is 485 mn/m. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. The model of a liquid surface acting like a stretched elastic sheet can effectively explain surface tension effects. Surface tension is responsible for the shape of. When a water droplet forms, it tends to take on a spherical shape due to surface tension. Suppose the cohesive forces are less than the adhesive forces. The water molecules on the surface of the droplet. In that case, the liquid will spread out, thus,. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it.
from www.slideserve.com
Surface tension is responsible for the shape of. The water molecules on the surface of the droplet. The model of a liquid surface acting like a stretched elastic sheet can effectively explain surface tension effects. In that case, the liquid will spread out, thus,. An example is mercury, whose surface tension at 25 °c is 485 mn/m. When a water droplet forms, it tends to take on a spherical shape due to surface tension. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. Suppose the cohesive forces are less than the adhesive forces. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or.
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free
Surface Tension Worked Examples Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. The water molecules on the surface of the droplet. In that case, the liquid will spread out, thus,. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. The model of a liquid surface acting like a stretched elastic sheet can effectively explain surface tension effects. When a water droplet forms, it tends to take on a spherical shape due to surface tension. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. An example is mercury, whose surface tension at 25 °c is 485 mn/m. Suppose the cohesive forces are less than the adhesive forces. Surface tension is responsible for the shape of.
From www.slideserve.com
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free Surface Tension Worked Examples The water molecules on the surface of the droplet. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. An example is mercury, whose surface tension at 25 °c is 485 mn/m. Suppose the cohesive forces are less than. Surface Tension Worked Examples.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Surface Tension Worked Examples In that case, the liquid will spread out, thus,. The model of a liquid surface acting like a stretched elastic sheet can effectively explain surface tension effects. Suppose the cohesive forces are less than the adhesive forces. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of. Surface Tension Worked Examples.
From askfilo.com
If surface tension is defined as force per unit length, which of the foll.. Surface Tension Worked Examples The model of a liquid surface acting like a stretched elastic sheet can effectively explain surface tension effects. Suppose the cohesive forces are less than the adhesive forces. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. In that case, the liquid will spread out, thus,.. Surface Tension Worked Examples.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Surface Tension Worked Examples The tendency to minimize that wall tension pulls the bubbles into spherical shapes. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. Suppose the cohesive forces are less than the adhesive forces. When a water droplet forms, it tends to take on a spherical shape due. Surface Tension Worked Examples.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit Surface Tension Worked Examples The tendency to minimize that wall tension pulls the bubbles into spherical shapes. Suppose the cohesive forces are less than the adhesive forces. An example is mercury, whose surface tension at 25 °c is 485 mn/m. In that case, the liquid will spread out, thus,. The water molecules on the surface of the droplet. Surface tension depends mainly upon the. Surface Tension Worked Examples.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Surface Tension Worked Examples When a water droplet forms, it tends to take on a spherical shape due to surface tension. Suppose the cohesive forces are less than the adhesive forces. The model of a liquid surface acting like a stretched elastic sheet can effectively explain surface tension effects. In that case, the liquid will spread out, thus,. The water molecules on the surface. Surface Tension Worked Examples.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Surface Tension Worked Examples Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Surface tension is responsible for the. Surface Tension Worked Examples.
From www.youtube.com
Surface Tension, part 2 Lecture 1.4 Chemical Engineering Fluid Surface Tension Worked Examples The water molecules on the surface of the droplet. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. Simply put, surface tension is the tendency of molecules of a liquid to be attracted. Surface Tension Worked Examples.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Surface Tension Worked Examples Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. When a water droplet forms, it tends to take on a spherical shape due to surface tension. Surface tension is responsible for the shape of. The tendency to minimize that wall tension pulls the bubbles into spherical. Surface Tension Worked Examples.
From www.youtube.com
Surface tension of water YouTube Surface Tension Worked Examples The tendency to minimize that wall tension pulls the bubbles into spherical shapes. The water molecules on the surface of the droplet. In that case, the liquid will spread out, thus,. When a water droplet forms, it tends to take on a spherical shape due to surface tension. Surface tension is responsible for the shape of. An example is mercury,. Surface Tension Worked Examples.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Surface Tension Worked Examples The model of a liquid surface acting like a stretched elastic sheet can effectively explain surface tension effects. In that case, the liquid will spread out, thus,. Surface tension is responsible for the shape of. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. An example. Surface Tension Worked Examples.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments Surface Tension Worked Examples In that case, the liquid will spread out, thus,. When a water droplet forms, it tends to take on a spherical shape due to surface tension. Suppose the cohesive forces are less than the adhesive forces. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a. Surface Tension Worked Examples.
From brainly.in
The dimensional formula for the surface tension? Brainly.in Surface Tension Worked Examples Surface tension is responsible for the shape of. The water molecules on the surface of the droplet. When a water droplet forms, it tends to take on a spherical shape due to surface tension. The model of a liquid surface acting like a stretched elastic sheet can effectively explain surface tension effects. Suppose the cohesive forces are less than the. Surface Tension Worked Examples.
From www.docdroid.net
Application_of_surface_tension_in_fluid_flow_1651146905.pdf DocDroid Surface Tension Worked Examples An example is mercury, whose surface tension at 25 °c is 485 mn/m. The model of a liquid surface acting like a stretched elastic sheet can effectively explain surface tension effects. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. In that case, the liquid will. Surface Tension Worked Examples.
From www.youtube.com
How does Surface Tension work? YouTube Surface Tension Worked Examples Suppose the cohesive forces are less than the adhesive forces. In that case, the liquid will spread out, thus,. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. The water molecules on the surface of the droplet. Surface. Surface Tension Worked Examples.
From www.youtube.com
Surface Tension of Water Explained YouTube Surface Tension Worked Examples In that case, the liquid will spread out, thus,. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. An example is mercury, whose surface tension at 25 °c is 485 mn/m. Surface tension is responsible for the shape of. When a water droplet forms, it tends to take on a spherical shape due to surface tension.. Surface Tension Worked Examples.
From www.youtube.com
Surface Tension YouTube Surface Tension Worked Examples The model of a liquid surface acting like a stretched elastic sheet can effectively explain surface tension effects. Surface tension is responsible for the shape of. When a water droplet forms, it tends to take on a spherical shape due to surface tension. The water molecules on the surface of the droplet. Surface tension depends mainly upon the forces of. Surface Tension Worked Examples.
From www.biolinscientific.com
Surface tension of water Why is it so high? Surface Tension Worked Examples When a water droplet forms, it tends to take on a spherical shape due to surface tension. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. The water molecules on the surface of. Surface Tension Worked Examples.
From www.pinterest.com
Pin on School! Surface Tension Worked Examples In that case, the liquid will spread out, thus,. The model of a liquid surface acting like a stretched elastic sheet can effectively explain surface tension effects. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. When a. Surface Tension Worked Examples.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download Surface Tension Worked Examples Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. An example is mercury, whose surface tension at 25 °c is 485 mn/m. Suppose the cohesive forces are less than the adhesive forces. When a water droplet forms, it tends to take on a spherical shape due. Surface Tension Worked Examples.
From www.purechemistry.org
SURFACE TENSION Purechemistry Surface Tension Worked Examples Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Surface tension is responsible for the shape of. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. Suppose the cohesive forces are less than the. Surface Tension Worked Examples.
From mungfali.com
Tension Diagram Surface Tension Worked Examples When a water droplet forms, it tends to take on a spherical shape due to surface tension. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. In that case, the liquid will spread out, thus,. The water molecules on the surface of the droplet. Suppose the. Surface Tension Worked Examples.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Surface Tension Worked Examples In that case, the liquid will spread out, thus,. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. When a water droplet forms, it tends to take on a spherical shape due to. Surface Tension Worked Examples.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces Surface Tension Worked Examples Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. The model of a liquid surface. Surface Tension Worked Examples.
From worksheetlibleglens.z13.web.core.windows.net
Tension And Surface Tension Surface Tension Worked Examples An example is mercury, whose surface tension at 25 °c is 485 mn/m. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Surface tension is responsible for the shape of. In that case, the liquid will spread out,. Surface Tension Worked Examples.
From byjus.com
Explain the surface tension phenomenon with examples. Surface Tension Worked Examples When a water droplet forms, it tends to take on a spherical shape due to surface tension. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. In that case, the liquid will spread out, thus,. The model of a liquid surface acting like a stretched elastic. Surface Tension Worked Examples.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Surface Tension Worked Examples Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Suppose the cohesive forces are less than the adhesive forces. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. The model of a liquid surface. Surface Tension Worked Examples.
From www.youtube.com
Cohesion, Adhesion, & Surface Tension YouTube Surface Tension Worked Examples The water molecules on the surface of the droplet. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. The model of a liquid surface acting like a stretched elastic sheet can effectively explain surface tension effects. An example is mercury, whose surface tension at 25 °c. Surface Tension Worked Examples.
From www.alamy.com
Thread and film surface tension experiment Stock Photo Alamy Surface Tension Worked Examples When a water droplet forms, it tends to take on a spherical shape due to surface tension. The water molecules on the surface of the droplet. Suppose the cohesive forces are less than the adhesive forces. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. An. Surface Tension Worked Examples.
From www.youtube.com
Surface Tension YouTube Surface Tension Worked Examples The tendency to minimize that wall tension pulls the bubbles into spherical shapes. An example is mercury, whose surface tension at 25 °c is 485 mn/m. When a water droplet forms, it tends to take on a spherical shape due to surface tension. The water molecules on the surface of the droplet. In that case, the liquid will spread out,. Surface Tension Worked Examples.
From carrotsareorange.com
Surface Tension Science Experiment for Kids Surface Tension Worked Examples Surface tension is responsible for the shape of. Suppose the cohesive forces are less than the adhesive forces. When a water droplet forms, it tends to take on a spherical shape due to surface tension. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. The model. Surface Tension Worked Examples.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Surface Tension Worked Examples An example is mercury, whose surface tension at 25 °c is 485 mn/m. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. When a water droplet forms, it tends to take on a spherical shape due to surface tension. Surface tension is responsible for the shape of. In that case, the liquid will spread out, thus,.. Surface Tension Worked Examples.
From readchemistry.com
Determination of Surface Tension Read Chemistry Surface Tension Worked Examples Suppose the cohesive forces are less than the adhesive forces. When a water droplet forms, it tends to take on a spherical shape due to surface tension. Surface tension is responsible for the shape of. An example is mercury, whose surface tension at 25 °c is 485 mn/m. Simply put, surface tension is the tendency of molecules of a liquid. Surface Tension Worked Examples.
From funsizephysics.com
What is Surface Tension? FunsizePhysics Surface Tension Worked Examples Suppose the cohesive forces are less than the adhesive forces. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. When a water droplet forms, it tends to take on a spherical shape due to surface tension. Simply put, surface tension is the tendency of molecules of. Surface Tension Worked Examples.
From www.youtube.com
The work done by surface tension on rising water do height of `h` in a Surface Tension Worked Examples Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. The water molecules on the surface of the droplet. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the. Surface Tension Worked Examples.