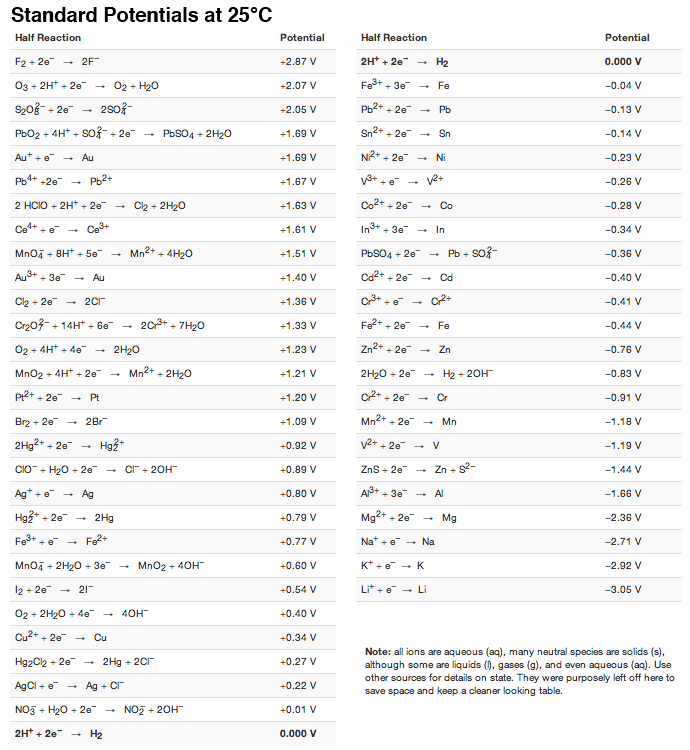
Standard Reduction Potential Of No3- . Values are from the following sources: the standard reduction potential can be determined by subtracting the standard reduction potential for the. standard reduction potentials table. reduction reactions in acidic solution are written using h + in place of h 3 o +. You may rewrite a reaction by. Standard electrode (reduction) potentials in aqueous solution at 25 °c. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. 183 rows the following table provides e o for selected reduction reactions. 47 rows the table of standard redox potentials for total and inorganic chemistry contains: the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard.
from rayb78.github.io
the standard reduction potential can be determined by subtracting the standard reduction potential for the. 47 rows the table of standard redox potentials for total and inorganic chemistry contains: Standard electrode (reduction) potentials in aqueous solution at 25 °c. You may rewrite a reaction by. Values are from the following sources: reduction reactions in acidic solution are written using h + in place of h 3 o +. standard reduction potentials table. 183 rows the following table provides e o for selected reduction reactions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard.
Standard Reduction Potentials Chart
Standard Reduction Potential Of No3- You may rewrite a reaction by. Values are from the following sources: 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. 183 rows the following table provides e o for selected reduction reactions. standard reduction potentials table. reduction reactions in acidic solution are written using h + in place of h 3 o +. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. the standard reduction potential can be determined by subtracting the standard reduction potential for the. Standard electrode (reduction) potentials in aqueous solution at 25 °c. You may rewrite a reaction by. 47 rows the table of standard redox potentials for total and inorganic chemistry contains:
From www.chegg.com
Solved Use the appropriate standard reduction Standard Reduction Potential Of No3- Standard electrode (reduction) potentials in aqueous solution at 25 °c. the standard reduction potential can be determined by subtracting the standard reduction potential for the. Values are from the following sources: the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. standard reduction potentials. Standard Reduction Potential Of No3-.
From hasyimmi.blogspot.com
Standard Reduction Potential Table Reduction Potentials in the Standard Reduction Potential Of No3- You may rewrite a reaction by. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Standard electrode (reduction) potentials in aqueous solution at 25 °c. the standard reduction potential can be determined by subtracting the standard reduction potential for the. 183 rows the following table provides. Standard Reduction Potential Of No3-.
From www.researchgate.net
Overview of the response of NH3 oxidation, NO3− reduction and anammox Standard Reduction Potential Of No3- the standard reduction potential can be determined by subtracting the standard reduction potential for the. 47 rows the table of standard redox potentials for total and inorganic chemistry contains: You may rewrite a reaction by. Values are from the following sources: the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction. Standard Reduction Potential Of No3-.
From chem.libretexts.org
17.3 Standard Reduction Potentials Chemistry LibreTexts Standard Reduction Potential Of No3- You may rewrite a reaction by. 47 rows the table of standard redox potentials for total and inorganic chemistry contains: the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. standard reduction potentials table. the standard reduction potential can be determined by subtracting. Standard Reduction Potential Of No3-.
From www.chegg.com
Solved I. Reduction Potential and Reaction Spontaneity B. Standard Reduction Potential Of No3- 183 rows the following table provides e o for selected reduction reactions. Standard electrode (reduction) potentials in aqueous solution at 25 °c. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. 47 rows the table of standard redox potentials for total and inorganic chemistry contains: . Standard Reduction Potential Of No3-.
From www.pinterest.co.uk
pe=log[e] measuved in volts.if pe is negative reduced, if pe is Standard Reduction Potential Of No3- reduction reactions in acidic solution are written using h + in place of h 3 o +. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. standard reduction potentials table. 372 rows the data below tabulates standard electrode potentials (e°), in volts. Standard Reduction Potential Of No3-.
From ch302.cm.utexas.edu
stdpotsshortlist.png Standard Reduction Potential Of No3- the standard reduction potential can be determined by subtracting the standard reduction potential for the. standard reduction potentials table. You may rewrite a reaction by. Values are from the following sources: the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. 372 rows. Standard Reduction Potential Of No3-.
From www.toppr.com
The standard reduction potential for the half cell NO3^(aq.) + 2H Standard Reduction Potential Of No3- the standard reduction potential can be determined by subtracting the standard reduction potential for the. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. reduction reactions in acidic solution are written using h + in place of h 3 o +. the standard reduction potential. Standard Reduction Potential Of No3-.
From ecampusontario.pressbooks.pub
Appendix 4 Standard Reduction Potentials First Year General Chemistry Standard Reduction Potential Of No3- Values are from the following sources: You may rewrite a reaction by. 183 rows the following table provides e o for selected reduction reactions. standard reduction potentials table. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Standard electrode (reduction) potentials in aqueous solution at 25. Standard Reduction Potential Of No3-.
From byjus.com
The standard reduction potential of the reaction at 25^° C ,Н2О + e Standard Reduction Potential Of No3- Values are from the following sources: standard reduction potentials table. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. 183 rows the following table provides e o for selected reduction reactions. 372 rows the data below tabulates standard electrode potentials (e°), in. Standard Reduction Potential Of No3-.
From www.chegg.com
Solved What is the standard emf of a galvanic cell made of a Standard Reduction Potential Of No3- You may rewrite a reaction by. Values are from the following sources: Standard electrode (reduction) potentials in aqueous solution at 25 °c. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. reduction reactions in acidic solution are written using h + in place of. Standard Reduction Potential Of No3-.
From www.pinterest.com
Standard Reduction Potential (E) when given two half reactions and Standard Reduction Potential Of No3- 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. You may rewrite a reaction by. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. standard reduction potentials table. reduction reactions in. Standard Reduction Potential Of No3-.
From www.bartleby.com
Answered TABLE 20.1 Standard Reduction… bartleby Standard Reduction Potential Of No3- 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. the standard reduction potential can be determined by subtracting the standard reduction potential for the. 183 rows the following table provides e o for selected reduction reactions. Values are from the following sources: Standard electrode (reduction) potentials. Standard Reduction Potential Of No3-.
From www.researchgate.net
Average values and standard deviation of pH, redox potential (Eh Standard Reduction Potential Of No3- 183 rows the following table provides e o for selected reduction reactions. reduction reactions in acidic solution are written using h + in place of h 3 o +. standard reduction potentials table. Standard electrode (reduction) potentials in aqueous solution at 25 °c. the standard reduction potential can be determined by subtracting the standard reduction potential. Standard Reduction Potential Of No3-.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Of No3- the standard reduction potential can be determined by subtracting the standard reduction potential for the. 183 rows the following table provides e o for selected reduction reactions. Standard electrode (reduction) potentials in aqueous solution at 25 °c. You may rewrite a reaction by. the standard reduction potential can be determined by subtracting the standard reduction potential for. Standard Reduction Potential Of No3-.
From chemwiki.ucdavis.edu
17.3 Standard Reduction Potentials Chemwiki Standard Reduction Potential Of No3- Values are from the following sources: the standard reduction potential can be determined by subtracting the standard reduction potential for the. standard reduction potentials table. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. Standard electrode (reduction) potentials in aqueous solution at 25. Standard Reduction Potential Of No3-.
From www.researchgate.net
What is the reduction potential of DNRA or nitrate ammonification? Standard Reduction Potential Of No3- the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. the standard reduction potential can be determined by subtracting the standard reduction potential for the. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she),. Standard Reduction Potential Of No3-.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Of No3- Standard electrode (reduction) potentials in aqueous solution at 25 °c. standard reduction potentials table. reduction reactions in acidic solution are written using h + in place of h 3 o +. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. You may rewrite a reaction by.. Standard Reduction Potential Of No3-.
From pubs.acs.org
Standard Reduction Potentials for Oxygen and Carbon Dioxide Couples in Standard Reduction Potential Of No3- the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. the standard reduction potential can be determined by subtracting the standard reduction potential for the. You may rewrite a reaction by. Standard electrode (reduction) potentials in aqueous solution at 25 °c. reduction reactions in. Standard Reduction Potential Of No3-.
From www.toppr.com
The standard reduction potential for the half cell NO3^(aq) + 2H Standard Reduction Potential Of No3- Standard electrode (reduction) potentials in aqueous solution at 25 °c. reduction reactions in acidic solution are written using h + in place of h 3 o +. Values are from the following sources: 183 rows the following table provides e o for selected reduction reactions. You may rewrite a reaction by. 372 rows the data below tabulates. Standard Reduction Potential Of No3-.
From www.tpsearchtool.com
Standard Reduction Potential Charts For Chemistry Images Standard Reduction Potential Of No3- 47 rows the table of standard redox potentials for total and inorganic chemistry contains: 183 rows the following table provides e o for selected reduction reactions. You may rewrite a reaction by. Standard electrode (reduction) potentials in aqueous solution at 25 °c. reduction reactions in acidic solution are written using h + in place of h 3. Standard Reduction Potential Of No3-.
From www.chegg.com
Solved Calculate the standard cell potential of the Standard Reduction Potential Of No3- reduction reactions in acidic solution are written using h + in place of h 3 o +. Values are from the following sources: Standard electrode (reduction) potentials in aqueous solution at 25 °c. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. the standard reduction potential. Standard Reduction Potential Of No3-.
From www.chegg.com
Solved Refer to the table of standard reduction potentials Standard Reduction Potential Of No3- Standard electrode (reduction) potentials in aqueous solution at 25 °c. reduction reactions in acidic solution are written using h + in place of h 3 o +. 47 rows the table of standard redox potentials for total and inorganic chemistry contains: the standard reduction potential can be determined by subtracting the standard reduction potential for the. . Standard Reduction Potential Of No3-.
From www.toppr.com
The standard reduction potential for the half cell NO3^(aq.) + 2H Standard Reduction Potential Of No3- You may rewrite a reaction by. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Values are from the following sources: 183 rows the following table provides e o for selected reduction reactions. the standard reduction potential can be determined by subtracting the standard reduction potential. Standard Reduction Potential Of No3-.
From www.numerade.com
SOLVED If the standard reduction potential, E" for the reduction of Standard Reduction Potential Of No3- reduction reactions in acidic solution are written using h + in place of h 3 o +. standard reduction potentials table. Standard electrode (reduction) potentials in aqueous solution at 25 °c. the standard reduction potential can be determined by subtracting the standard reduction potential for the. Values are from the following sources: the standard reduction potential. Standard Reduction Potential Of No3-.
From www.pveducation.org
Standard Potential PVEducation Standard Reduction Potential Of No3- reduction reactions in acidic solution are written using h + in place of h 3 o +. the standard reduction potential can be determined by subtracting the standard reduction potential for the. 183 rows the following table provides e o for selected reduction reactions. Standard electrode (reduction) potentials in aqueous solution at 25 °c. the standard. Standard Reduction Potential Of No3-.
From byjus.com
The s†an ded reduction potential for the half cell NO3 (aq) + 2H+(aq Standard Reduction Potential Of No3- standard reduction potentials table. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. reduction reactions in acidic solution are written using h + in place of h 3 o +. Standard electrode (reduction) potentials in aqueous solution at 25 °c. the standard reduction potential can. Standard Reduction Potential Of No3-.
From vpia.org.vn
Standard Reduction Potentials made easy, reduction line Standard Reduction Potential Of No3- 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. 183 rows the following table provides e o for selected reduction reactions. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. standard. Standard Reduction Potential Of No3-.
From oneclass.com
OneClass Given the following halfreactions and their respective Standard Reduction Potential Of No3- the standard reduction potential can be determined by subtracting the standard reduction potential for the. reduction reactions in acidic solution are written using h + in place of h 3 o +. Values are from the following sources: Standard electrode (reduction) potentials in aqueous solution at 25 °c. the standard reduction potential can be determined by subtracting. Standard Reduction Potential Of No3-.
From exodxlskk.blob.core.windows.net
Standard Reduction Potential Of Zn2+/Zn at James Tarver blog Standard Reduction Potential Of No3- Standard electrode (reduction) potentials in aqueous solution at 25 °c. the standard reduction potential can be determined by subtracting the standard reduction potential for the. 47 rows the table of standard redox potentials for total and inorganic chemistry contains: Values are from the following sources: 372 rows the data below tabulates standard electrode potentials (e°), in volts. Standard Reduction Potential Of No3-.
From www.chegg.com
Solved TABLE 14.1 "Electron Tower" of Standard Reduction Standard Reduction Potential Of No3- You may rewrite a reaction by. the standard reduction potential can be determined by subtracting the standard reduction potential for the. 183 rows the following table provides e o for selected reduction reactions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. 47 rows the. Standard Reduction Potential Of No3-.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials Chemistry Standard Reduction Potential Of No3- Standard electrode (reduction) potentials in aqueous solution at 25 °c. the standard reduction potential can be determined by subtracting the standard reduction potential for the. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. standard reduction potentials table. 183 rows the following table provides e. Standard Reduction Potential Of No3-.
From www.chegg.com
Solved Using Table 14.1 (below) calculate the standard Standard Reduction Potential Of No3- 47 rows the table of standard redox potentials for total and inorganic chemistry contains: 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. reduction reactions in acidic solution are written using h + in place of h 3 o +. 183 rows the following table. Standard Reduction Potential Of No3-.
From www.toppr.com
The standard reduction potential for the half cell NO3^(aq) + 2H Standard Reduction Potential Of No3- 47 rows the table of standard redox potentials for total and inorganic chemistry contains: Standard electrode (reduction) potentials in aqueous solution at 25 °c. reduction reactions in acidic solution are written using h + in place of h 3 o +. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction. Standard Reduction Potential Of No3-.
From byjus.com
1. A galvanic cell is set up with a copper electrode in contact with 0 Standard Reduction Potential Of No3- standard reduction potentials table. 183 rows the following table provides e o for selected reduction reactions. Standard electrode (reduction) potentials in aqueous solution at 25 °c. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. 372 rows the data below tabulates standard. Standard Reduction Potential Of No3-.