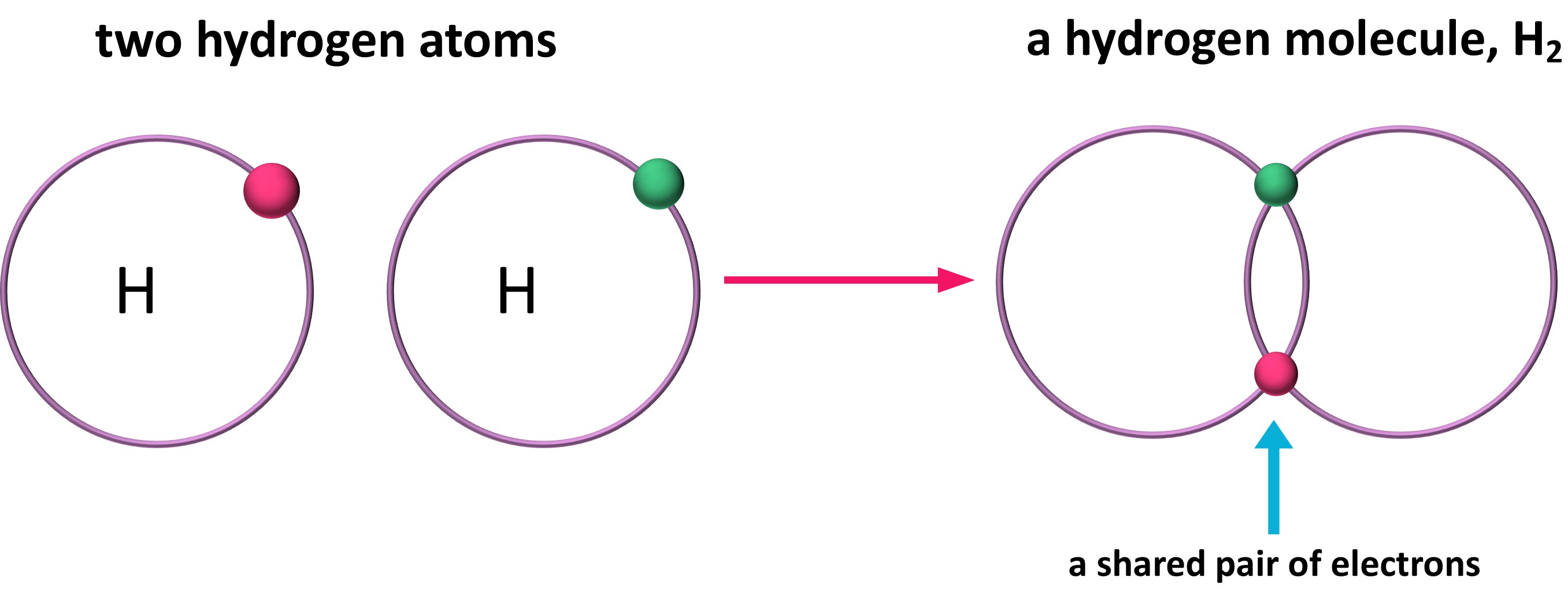
How Is Water Covalently Bonded . Learn the basics about the covalent bonding of water, when learning about covalent. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. A covalent bond involves the sharing of electron pairs between atoms. An oxygen atom has 6 electrons in its outer shell. What is a water molecule? Bonds between two nonmetals are generally covalent; Covalent compounds are insoluble in polar solvents like water. The binding arises from the electrostatic. Bonding between a metal and a nonmetal is often ionic. However, they dissolve in nonpolar solvents like benzene and toluene. A covalent bond can be classified by the number of. These electron pairs are known as shared pairs of electrons. Oxygen is in group 6 of the periodic table.
from thesciencecore.blogspot.com
The binding arises from the electrostatic. A covalent bond can be classified by the number of. Bonding between a metal and a nonmetal is often ionic. A covalent bond involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs of electrons. Covalent compounds are insoluble in polar solvents like water. What is a water molecule? Learn the basics about the covalent bonding of water, when learning about covalent. Bonds between two nonmetals are generally covalent; An oxygen atom has 6 electrons in its outer shell.
covalent bond definition, properties and examples The Science Core
How Is Water Covalently Bonded What is a water molecule? Bonds between two nonmetals are generally covalent; Bonding between a metal and a nonmetal is often ionic. However, they dissolve in nonpolar solvents like benzene and toluene. Oxygen is in group 6 of the periodic table. Covalent compounds are insoluble in polar solvents like water. What is a water molecule? These electron pairs are known as shared pairs of electrons. An oxygen atom has 6 electrons in its outer shell. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. A covalent bond can be classified by the number of. A covalent bond involves the sharing of electron pairs between atoms. The binding arises from the electrostatic. Learn the basics about the covalent bonding of water, when learning about covalent.
From www.science-revision.co.uk
Covalent bonding How Is Water Covalently Bonded Learn the basics about the covalent bonding of water, when learning about covalent. Oxygen is in group 6 of the periodic table. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. These electron pairs are known as shared pairs of electrons. A covalent bond can be classified by the number. How Is Water Covalently Bonded.
From philschatz.com
Chemical Bonds · Anatomy and Physiology How Is Water Covalently Bonded Oxygen is in group 6 of the periodic table. Bonding between a metal and a nonmetal is often ionic. A covalent bond involves the sharing of electron pairs between atoms. Learn the basics about the covalent bonding of water, when learning about covalent. An oxygen atom has 6 electrons in its outer shell. Covalent compounds are insoluble in polar solvents. How Is Water Covalently Bonded.
From www.britannica.com
covalent bond Definition, Properties, Examples, & Facts Britannica How Is Water Covalently Bonded What is a water molecule? Bonds between two nonmetals are generally covalent; However, they dissolve in nonpolar solvents like benzene and toluene. Covalent compounds are insoluble in polar solvents like water. A covalent bond involves the sharing of electron pairs between atoms. Oxygen is in group 6 of the periodic table. An oxygen atom has 6 electrons in its outer. How Is Water Covalently Bonded.
From www.biologyonline.com
Covalent bond Definition and Examples Biology Online Dictionary How Is Water Covalently Bonded These electron pairs are known as shared pairs of electrons. However, they dissolve in nonpolar solvents like benzene and toluene. Covalent compounds are insoluble in polar solvents like water. An oxygen atom has 6 electrons in its outer shell. Bonding between a metal and a nonmetal is often ionic. What is a water molecule? Oxygen is in group 6 of. How Is Water Covalently Bonded.
From www.dreamstime.com
H2O Covalent Bonding . Water Formula Diagram Design for Chemistry Labs How Is Water Covalently Bonded A covalent bond involves the sharing of electron pairs between atoms. The binding arises from the electrostatic. An oxygen atom has 6 electrons in its outer shell. Covalent compounds are insoluble in polar solvents like water. These electron pairs are known as shared pairs of electrons. Bonds between two nonmetals are generally covalent; A covalent bond can be classified by. How Is Water Covalently Bonded.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts How Is Water Covalently Bonded Oxygen is in group 6 of the periodic table. An oxygen atom has 6 electrons in its outer shell. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. A covalent bond involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs of electrons. Bonding. How Is Water Covalently Bonded.
From www.thoughtco.com
Is Water a Compound or an Element? How Is Water Covalently Bonded Learn the basics about the covalent bonding of water, when learning about covalent. However, they dissolve in nonpolar solvents like benzene and toluene. Oxygen is in group 6 of the periodic table. Bonding between a metal and a nonmetal is often ionic. What is a water molecule? A covalent bond involves the sharing of electron pairs between atoms. Covalent bond,. How Is Water Covalently Bonded.
From bio1151.nicerweb.com
water_molecule.html 03_02WaterMolecules_L.jpg How Is Water Covalently Bonded What is a water molecule? A covalent bond involves the sharing of electron pairs between atoms. Oxygen is in group 6 of the periodic table. The binding arises from the electrostatic. A covalent bond can be classified by the number of. An oxygen atom has 6 electrons in its outer shell. However, they dissolve in nonpolar solvents like benzene and. How Is Water Covalently Bonded.
From www.thoughtco.com
Examples of Covalent Bonds and Compounds How Is Water Covalently Bonded Covalent compounds are insoluble in polar solvents like water. An oxygen atom has 6 electrons in its outer shell. Bonds between two nonmetals are generally covalent; Oxygen is in group 6 of the periodic table. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. These electron pairs are known as. How Is Water Covalently Bonded.
From thesciencecore.blogspot.com
covalent bond definition, properties and examples The Science Core How Is Water Covalently Bonded Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. Learn the basics about the covalent bonding of water, when learning about covalent. Bonds between two nonmetals are generally covalent; A covalent bond can be classified by the number of. Oxygen is in group 6 of the periodic table. These electron. How Is Water Covalently Bonded.
From www.thoughtco.com
Covalent Bond Definition and Example (Chemistry) How Is Water Covalently Bonded These electron pairs are known as shared pairs of electrons. A covalent bond involves the sharing of electron pairs between atoms. An oxygen atom has 6 electrons in its outer shell. Bonds between two nonmetals are generally covalent; However, they dissolve in nonpolar solvents like benzene and toluene. Bonding between a metal and a nonmetal is often ionic. What is. How Is Water Covalently Bonded.
From mavink.com
Covalent Bonding Diagram How Is Water Covalently Bonded An oxygen atom has 6 electrons in its outer shell. However, they dissolve in nonpolar solvents like benzene and toluene. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. Learn the basics about the covalent bonding of water, when learning about covalent. Covalent compounds are insoluble in polar solvents like. How Is Water Covalently Bonded.
From thescienceandmathszone.com
Covalent Bonding The Science and Maths Zone How Is Water Covalently Bonded Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. However, they dissolve in nonpolar solvents like benzene and toluene. A covalent bond can be classified by the number of. An oxygen atom has 6 electrons in its outer shell. Covalent compounds are insoluble in polar solvents like water. Oxygen is. How Is Water Covalently Bonded.
From www.science-revision.co.uk
Covalent bonding How Is Water Covalently Bonded A covalent bond involves the sharing of electron pairs between atoms. Bonds between two nonmetals are generally covalent; Bonding between a metal and a nonmetal is often ionic. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. These electron pairs are known as shared pairs of electrons. However, they dissolve. How Is Water Covalently Bonded.
From mungfali.com
Water Polar Covalent Bond How Is Water Covalently Bonded Bonds between two nonmetals are generally covalent; Bonding between a metal and a nonmetal is often ionic. An oxygen atom has 6 electrons in its outer shell. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. Covalent compounds are insoluble in polar solvents like water. Oxygen is in group 6. How Is Water Covalently Bonded.
From www.britannica.com
chemical bonding Definition, Types, & Examples Britannica How Is Water Covalently Bonded Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. A covalent bond involves the sharing of electron pairs between atoms. However, they dissolve in nonpolar solvents like benzene and toluene. Bonds between two nonmetals are generally covalent; Learn the basics about the covalent bonding of water, when learning about covalent.. How Is Water Covalently Bonded.
From slideplayer.com
Chapter 12 Covalent Bonding ppt download How Is Water Covalently Bonded Bonding between a metal and a nonmetal is often ionic. The binding arises from the electrostatic. Learn the basics about the covalent bonding of water, when learning about covalent. However, they dissolve in nonpolar solvents like benzene and toluene. Oxygen is in group 6 of the periodic table. These electron pairs are known as shared pairs of electrons. A covalent. How Is Water Covalently Bonded.
From sciencenotes.org
Covalent Compounds Examples and Properties How Is Water Covalently Bonded Bonding between a metal and a nonmetal is often ionic. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. Bonds between two nonmetals are generally covalent; However, they dissolve in nonpolar solvents like benzene and toluene. A covalent bond involves the sharing of electron pairs between atoms. Oxygen is in. How Is Water Covalently Bonded.
From www.tec-science.com
Covalent bonding tecscience How Is Water Covalently Bonded What is a water molecule? The binding arises from the electrostatic. An oxygen atom has 6 electrons in its outer shell. A covalent bond involves the sharing of electron pairs between atoms. A covalent bond can be classified by the number of. Bonding between a metal and a nonmetal is often ionic. Covalent bond, in chemistry, the interatomic linkage that. How Is Water Covalently Bonded.
From www.ck12.org
License CC BYNC 3.0 How Is Water Covalently Bonded Bonding between a metal and a nonmetal is often ionic. An oxygen atom has 6 electrons in its outer shell. A covalent bond can be classified by the number of. A covalent bond involves the sharing of electron pairs between atoms. However, they dissolve in nonpolar solvents like benzene and toluene. What is a water molecule? Covalent compounds are insoluble. How Is Water Covalently Bonded.
From biologydictionary.net
Covalent Bond Biology Dictionary How Is Water Covalently Bonded Bonds between two nonmetals are generally covalent; These electron pairs are known as shared pairs of electrons. The binding arises from the electrostatic. A covalent bond can be classified by the number of. Bonding between a metal and a nonmetal is often ionic. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between. How Is Water Covalently Bonded.
From www.alamy.com
Diagram to illustrate covalent bonding in water with a fully labelled How Is Water Covalently Bonded Bonding between a metal and a nonmetal is often ionic. These electron pairs are known as shared pairs of electrons. A covalent bond involves the sharing of electron pairs between atoms. The binding arises from the electrostatic. Covalent compounds are insoluble in polar solvents like water. A covalent bond can be classified by the number of. However, they dissolve in. How Is Water Covalently Bonded.
From www.biologybrain.com
If two covalently bonded atoms are identical the bond is? Biology Brain How Is Water Covalently Bonded Bonding between a metal and a nonmetal is often ionic. These electron pairs are known as shared pairs of electrons. The binding arises from the electrostatic. What is a water molecule? Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. Learn the basics about the covalent bonding of water, when. How Is Water Covalently Bonded.
From www2.victoriacollege.edu
polar covalent bonds of water How Is Water Covalently Bonded However, they dissolve in nonpolar solvents like benzene and toluene. A covalent bond can be classified by the number of. Learn the basics about the covalent bonding of water, when learning about covalent. The binding arises from the electrostatic. Covalent compounds are insoluble in polar solvents like water. What is a water molecule? An oxygen atom has 6 electrons in. How Is Water Covalently Bonded.
From overallscience.com
Covalent bond (covalency) and its type Overall Science How Is Water Covalently Bonded An oxygen atom has 6 electrons in its outer shell. The binding arises from the electrostatic. A covalent bond can be classified by the number of. Learn the basics about the covalent bonding of water, when learning about covalent. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. However, they. How Is Water Covalently Bonded.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID588326 How Is Water Covalently Bonded Bonding between a metal and a nonmetal is often ionic. The binding arises from the electrostatic. What is a water molecule? Covalent compounds are insoluble in polar solvents like water. An oxygen atom has 6 electrons in its outer shell. Oxygen is in group 6 of the periodic table. A covalent bond can be classified by the number of. However,. How Is Water Covalently Bonded.
From www.chemistrylearner.com
Covalent Bond Definition, Types, and Examples How Is Water Covalently Bonded Learn the basics about the covalent bonding of water, when learning about covalent. These electron pairs are known as shared pairs of electrons. The binding arises from the electrostatic. Oxygen is in group 6 of the periodic table. An oxygen atom has 6 electrons in its outer shell. Covalent bond, in chemistry, the interatomic linkage that results from the sharing. How Is Water Covalently Bonded.
From www.animalia-life.club
Bond Covalent Bond In Water How Is Water Covalently Bonded What is a water molecule? Covalent compounds are insoluble in polar solvents like water. Bonds between two nonmetals are generally covalent; Bonding between a metal and a nonmetal is often ionic. However, they dissolve in nonpolar solvents like benzene and toluene. Learn the basics about the covalent bonding of water, when learning about covalent. A covalent bond involves the sharing. How Is Water Covalently Bonded.
From oerpub.github.io
This figure shows three water molecules and the hydrogen bonds between How Is Water Covalently Bonded These electron pairs are known as shared pairs of electrons. Oxygen is in group 6 of the periodic table. What is a water molecule? A covalent bond involves the sharing of electron pairs between atoms. The binding arises from the electrostatic. However, they dissolve in nonpolar solvents like benzene and toluene. Covalent compounds are insoluble in polar solvents like water.. How Is Water Covalently Bonded.
From slideplayer.com
Structure of Water H & O covalently bonded together. H & O covalently How Is Water Covalently Bonded Oxygen is in group 6 of the periodic table. Bonding between a metal and a nonmetal is often ionic. Bonds between two nonmetals are generally covalent; These electron pairs are known as shared pairs of electrons. What is a water molecule? An oxygen atom has 6 electrons in its outer shell. Covalent bond, in chemistry, the interatomic linkage that results. How Is Water Covalently Bonded.
From www.priyamstudycentre.com
Covalent Bond Types, Definition, Properties, Examples How Is Water Covalently Bonded Covalent compounds are insoluble in polar solvents like water. However, they dissolve in nonpolar solvents like benzene and toluene. Learn the basics about the covalent bonding of water, when learning about covalent. What is a water molecule? Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. Bonding between a metal. How Is Water Covalently Bonded.
From mungfali.com
Water Molecules Covalent And Hydrogen Bonds How Is Water Covalently Bonded A covalent bond can be classified by the number of. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. Bonding between a metal and a nonmetal is often ionic. Covalent compounds are insoluble in polar solvents like water. An oxygen atom has 6 electrons in its outer shell. The binding. How Is Water Covalently Bonded.
From ecampusontario.pressbooks.pub
11.2 Covalent Bonding Chemistry v. 1 backup How Is Water Covalently Bonded A covalent bond involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs of electrons. A covalent bond can be classified by the number of. Covalent compounds are insoluble in polar solvents like water. Bonding between a metal and a nonmetal is often ionic. What is a water molecule? Bonds between two nonmetals are. How Is Water Covalently Bonded.
From surfguppy.com
What is Polar Covalent Bond How Is Water Covalently Bonded Covalent compounds are insoluble in polar solvents like water. Learn the basics about the covalent bonding of water, when learning about covalent. These electron pairs are known as shared pairs of electrons. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. The binding arises from the electrostatic. A covalent bond. How Is Water Covalently Bonded.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk How Is Water Covalently Bonded A covalent bond involves the sharing of electron pairs between atoms. Learn the basics about the covalent bonding of water, when learning about covalent. Oxygen is in group 6 of the periodic table. These electron pairs are known as shared pairs of electrons. Covalent compounds are insoluble in polar solvents like water. Bonds between two nonmetals are generally covalent; The. How Is Water Covalently Bonded.