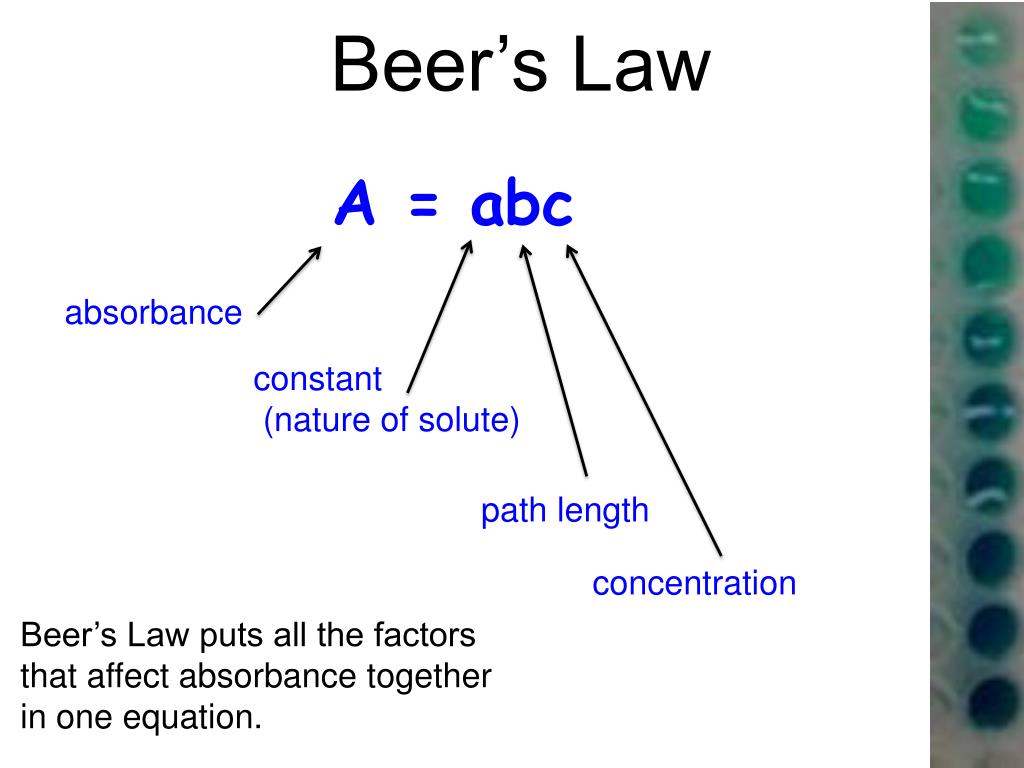
What Is Beer's Law Quizlet . There are two contributions to this fundamental. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Beer’s law is a limiting law that is valid only for low concentrations of analyte. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more. The premise is that a light beam becomes weaker as. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Absorbance is directly proportional to the concentration of a solution. If you plot absorbance vs concentration, the resulting. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In other words, a solution.
from www.slideserve.com
Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Absorbance is directly proportional to the concentration of a solution. Beer’s law is a limiting law that is valid only for low concentrations of analyte. Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise is that a light beam becomes weaker as. If you plot absorbance vs concentration, the resulting. In other words, a solution. There are two contributions to this fundamental.
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download ID2658679
What Is Beer's Law Quizlet Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more. Absorbance is directly proportional to the concentration of a solution. In other words, a solution. The premise is that a light beam becomes weaker as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. If you plot absorbance vs concentration, the resulting. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. There are two contributions to this fundamental. Beer’s law is a limiting law that is valid only for low concentrations of analyte. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more.
From www.studypool.com
SOLUTION Beer S Law Answer Studypool What Is Beer's Law Quizlet In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. There are two contributions to this fundamental. If you plot absorbance vs concentration, the resulting. In other words, a solution. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. What Is Beer's Law Quizlet.
From www.youtube.com
BeerLambert law in easy way YouTube What Is Beer's Law Quizlet \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. In other words, a solution. If you plot absorbance vs concentration, the resulting. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. There are two contributions to this fundamental. In spectroscopy, beer’s law states that the absorption. What Is Beer's Law Quizlet.
From www.youtube.com
Derivation of Beer Lambert Law YouTube What Is Beer's Law Quizlet In other words, a solution. Absorbance is directly proportional to the concentration of a solution. There are two contributions to this fundamental. The premise is that a light beam becomes weaker as. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Since the concentration,. What Is Beer's Law Quizlet.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations What Is Beer's Law Quizlet Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise is that a light beam becomes weaker as. Absorbance is directly proportional to the concentration of a solution. Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more. Beer’s law is a limiting. What Is Beer's Law Quizlet.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download ID3102589 What Is Beer's Law Quizlet Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more. The premise is that a light beam becomes weaker as. Beer's law states that a. What Is Beer's Law Quizlet.
From facts.net
13 Surprising Facts About BeerLambert Law What Is Beer's Law Quizlet \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Absorbance is directly proportional to the concentration of a solution. There are two contributions to this fundamental. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution. Study with quizlet and memorize flashcards. What Is Beer's Law Quizlet.
From openlab.citytech.cuny.edu
Beer’s Law Biology 1101 Course Hub What Is Beer's Law Quizlet In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. There are two contributions to this fundamental. In other words, a solution. The premise is that a light beam becomes. What Is Beer's Law Quizlet.
From www.youtube.com
Analytical Instrumentation Tutorial 2 Beer Lambert Law YouTube What Is Beer's Law Quizlet If you plot absorbance vs concentration, the resulting. The premise is that a light beam becomes weaker as. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer’s law is a limiting law that is valid only for low concentrations of analyte. Since the. What Is Beer's Law Quizlet.
From chemistrysources.com
قانون بيير (بير) Beer’s Law التعريف و المعادلة مصادر الكيمياء What Is Beer's Law Quizlet Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more. Beer’s law is a limiting law that is valid only for low concentrations of analyte. In other words, a solution. If you plot absorbance vs concentration, the resulting. There are two contributions to this fundamental. Since the concentration, path length. What Is Beer's Law Quizlet.
From lelandchemclub.weebly.com
Solutions Leland Chemistry Club What Is Beer's Law Quizlet If you plot absorbance vs concentration, the resulting. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more. Absorbance is directly proportional to the concentration. What Is Beer's Law Quizlet.
From sciencenotes.org
Beer's Law Equation and Example What Is Beer's Law Quizlet Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Study with quizlet and memorize flashcards containing terms like what is beer's law?,. What Is Beer's Law Quizlet.
From lelandchemclub.weebly.com
Solutions Leland Chemistry Club What Is Beer's Law Quizlet The premise is that a light beam becomes weaker as. Beer’s law is a limiting law that is valid only for low concentrations of analyte. Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption.. What Is Beer's Law Quizlet.
From www.chegg.com
Solved A Beer's Law plot is shown here along with the What Is Beer's Law Quizlet In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. If you plot absorbance vs concentration, the resulting. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. There are two contributions to this fundamental. In other words, a solution.. What Is Beer's Law Quizlet.
From www.reddit.com
Visualization of Beer’s law in beer r/chemistry What Is Beer's Law Quizlet Beer’s law is a limiting law that is valid only for low concentrations of analyte. Absorbance is directly proportional to the concentration of a solution. There are two contributions to this fundamental. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Study with quizlet. What Is Beer's Law Quizlet.
From www.numerade.com
SOLVED Consider the Beer's Law equation A = εbc. Match the variable with the correct What Is Beer's Law Quizlet Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In other words, a solution. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. What Is Beer's Law Quizlet.
From study.com
Quiz & Worksheet Spectrophotometry & Beer's Law What Is Beer's Law Quizlet Beer’s law is a limiting law that is valid only for low concentrations of analyte. The premise is that a light beam becomes weaker as. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In spectroscopy, beer’s law states that the absorption of light. What Is Beer's Law Quizlet.
From www.thoughtco.com
Beer's Law Definition and Equation What Is Beer's Law Quizlet In other words, a solution. If you plot absorbance vs concentration, the resulting. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The premise is that a light beam becomes weaker as. Absorbance is directly proportional to the concentration of a solution. There are. What Is Beer's Law Quizlet.
From www.youtube.com
Beer's Law Overview YouTube What Is Beer's Law Quizlet There are two contributions to this fundamental. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Absorbance is directly proportional to the concentration of a solution. Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є). What Is Beer's Law Quizlet.
From www.coursehero.com
[Solved] What is the LambertBeer Law and how can you use it to... Course Hero What Is Beer's Law Quizlet Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. The premise is that a light beam becomes weaker as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In other words,. What Is Beer's Law Quizlet.
From www.chegg.com
Solved 2. (3 pts) What is the equation for Beer's Law? Why What Is Beer's Law Quizlet Beer’s law is a limiting law that is valid only for low concentrations of analyte. The premise is that a light beam becomes weaker as. There are two contributions to this fundamental. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Absorbance is directly. What Is Beer's Law Quizlet.
From www.studocu.com
Beers Law Lab Ph ET Student Sheet AP Chemistry PhET Simulation Beer’s Law Lab Learning Goals What Is Beer's Law Quizlet Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more. Beer’s law is a limiting law that is valid only for low concentrations of analyte. In other words, a solution. The premise is that a light beam becomes weaker as. If you plot absorbance vs concentration, the resulting. There are. What Is Beer's Law Quizlet.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry, Basic Introduction What Is Beer's Law Quizlet Absorbance is directly proportional to the concentration of a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The premise is that a light beam becomes weaker as. Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law,. What Is Beer's Law Quizlet.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download ID2658679 What Is Beer's Law Quizlet Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more. Absorbance is directly proportional to the concentration of a solution. There are two contributions to this fundamental. If you plot absorbance vs concentration, the resulting.. What Is Beer's Law Quizlet.
From www.studocu.com
Chem 181 4 Beer's Law Beer Lab Pre lab Determining the Concentration of a Solution Beer’s What Is Beer's Law Quizlet In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption.. What Is Beer's Law Quizlet.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 What Is Beer's Law Quizlet \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Absorbance is directly proportional to the concentration of a solution. Beer’s law is a limiting law that is valid only for low concentrations of analyte. If you plot absorbance vs concentration, the resulting. Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more.. What Is Beer's Law Quizlet.
From www.chegg.com
Solved Beer's Law PRELAB ASSIGNMENT This experiment covers What Is Beer's Law Quizlet Absorbance is directly proportional to the concentration of a solution. In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more.. What Is Beer's Law Quizlet.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download ID2658679 What Is Beer's Law Quizlet Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption.. What Is Beer's Law Quizlet.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation What Is Beer's Law Quizlet \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. The premise is that a light beam becomes weaker as. Absorbance is directly proportional to the concentration of a solution. Beer’s law is a limiting law that is valid only for low concentrations of analyte. If you plot absorbance vs concentration, the resulting. Since the concentration, path length and molar absorptivity are all. What Is Beer's Law Quizlet.
From www.chegg.com
Solved PreLab Assignment Beer's Law This experiment covers What Is Beer's Law Quizlet Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. If you plot absorbance vs concentration, the resulting. In spectroscopy, beer’s law states. What Is Beer's Law Quizlet.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples What Is Beer's Law Quizlet In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Absorbance is directly proportional to the concentration of a solution. In other words, a solution. The premise is that a light beam becomes weaker as. There are two contributions to this fundamental. \[\log \left( \frac{i_{o}}{i}. What Is Beer's Law Quizlet.
From www.youtube.com
Beer's Law Concept, Calculations, & Lab YouTube What Is Beer's Law Quizlet In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer’s law is a limiting law that is valid only for low concentrations of analyte. The premise is that a light beam becomes weaker as. There are two contributions to this fundamental. Beer's law states. What Is Beer's Law Quizlet.
From www.slideserve.com
PPT LAB 3 PowerPoint Presentation, free download ID2225939 What Is Beer's Law Quizlet Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law, molar absorptivity (є) and more. In other words, a solution. Absorbance is directly proportional to the concentration of a solution. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. There are two contributions to this fundamental. Since the concentration, path length and molar absorptivity are all directly. What Is Beer's Law Quizlet.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 from Advanced Chemistry What Is Beer's Law Quizlet Beer’s law is a limiting law that is valid only for low concentrations of analyte. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In other words, a solution. Absorbance is directly proportional to the concentration of a solution. Study with quizlet and memorize flashcards containing terms like what is beer's law?, beer's law,. What Is Beer's Law Quizlet.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative What Is Beer's Law Quizlet Beer’s law is a limiting law that is valid only for low concentrations of analyte. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. What Is Beer's Law Quizlet.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law PowerPoint Presentation ID5611981 What Is Beer's Law Quizlet \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. The premise is that a light beam becomes weaker as. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. There are two contributions to this fundamental. Since the concentration, path length and molar absorptivity are all directly. What Is Beer's Law Quizlet.