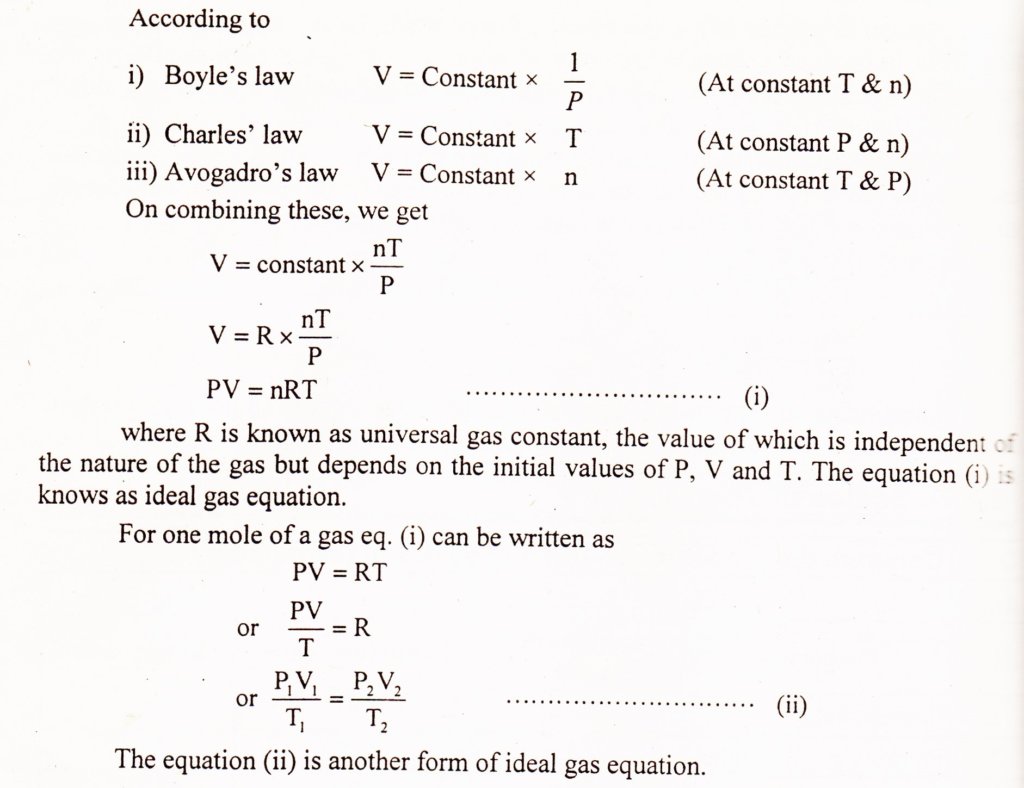
How To Use The Ideal Gas Law Equation . Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number. uses of the ideal gas law including solving for an unknown variable, comparing initial and final states, and finding partial pressure. Molecular collisions within a closed container (a propane tank) are shown (right). use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. The arrows represent the random. (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. in such a case, all gases obey an equation of state known as the ideal gas law: we can use the ideal gas equation to calculate the volume of 1 mole of an ideal gas at 0°c and 1 atmosphere pressure.
from chemistryskills.com
The arrows represent the random. (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number. in such a case, all gases obey an equation of state known as the ideal gas law: Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. Molecular collisions within a closed container (a propane tank) are shown (right). uses of the ideal gas law including solving for an unknown variable, comparing initial and final states, and finding partial pressure. use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. we can use the ideal gas equation to calculate the volume of 1 mole of an ideal gas at 0°c and 1 atmosphere pressure.
Ideal Gas Law Combined Gas Law Chemistry Skills
How To Use The Ideal Gas Law Equation (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) uses of the ideal gas law including solving for an unknown variable, comparing initial and final states, and finding partial pressure. The arrows represent the random. in such a case, all gases obey an equation of state known as the ideal gas law: Molecular collisions within a closed container (a propane tank) are shown (right). use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number. use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. we can use the ideal gas equation to calculate the volume of 1 mole of an ideal gas at 0°c and 1 atmosphere pressure.
From abiviralzz.blogspot.com
Worksheet On Ideal Gas Equation Ideal Gas Law Explained Ideal gas How To Use The Ideal Gas Law Equation use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. the ideal gas law is derived. How To Use The Ideal Gas Law Equation.
From www.britannica.com
Ideal gas Definition, Equation, Properties, & Facts Britannica How To Use The Ideal Gas Law Equation we can use the ideal gas equation to calculate the volume of 1 mole of an ideal gas at 0°c and 1 atmosphere pressure. Molecular collisions within a closed container (a propane tank) are shown (right). in such a case, all gases obey an equation of state known as the ideal gas law: The arrows represent the random.. How To Use The Ideal Gas Law Equation.
From www.youtube.com
Ideal Gas Equation How to Choose the Correct Gas Constant, R? With How To Use The Ideal Gas Law Equation uses of the ideal gas law including solving for an unknown variable, comparing initial and final states, and finding partial pressure. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. Molecular collisions within a closed container (a propane tank) are. How To Use The Ideal Gas Law Equation.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID How To Use The Ideal Gas Law Equation The arrows represent the random. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. Molecular collisions within a closed container (a propane tank) are shown (right). we can use the ideal gas equation to calculate the volume of 1 mole. How To Use The Ideal Gas Law Equation.
From sciencenotes.org
Ideal Gas Law Formula and Examples How To Use The Ideal Gas Law Equation Molecular collisions within a closed container (a propane tank) are shown (right). use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) the ideal gas law is derived from. How To Use The Ideal Gas Law Equation.
From www.youtube.com
Ideal Gas Law Practice Problems YouTube How To Use The Ideal Gas Law Equation the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number. The arrows represent the random. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. (the universal gas constant is defined as avogadro’s number na times the. How To Use The Ideal Gas Law Equation.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download How To Use The Ideal Gas Law Equation use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number. in. How To Use The Ideal Gas Law Equation.
From chemistryguru.com.sg
Ideal Gas Law and Applications How To Use The Ideal Gas Law Equation The arrows represent the random. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. in such a case, all gases obey an equation of state known as the ideal gas law: use the ideal gas equation to solve a problem when the amount of. How To Use The Ideal Gas Law Equation.
From www.youtube.com
Using the Ideal Gas Law to find density or molar mass YouTube How To Use The Ideal Gas Law Equation use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. The arrows represent the random. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. (the universal gas constant. How To Use The Ideal Gas Law Equation.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID How To Use The Ideal Gas Law Equation in such a case, all gases obey an equation of state known as the ideal gas law: (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) The arrows represent the random. we can use the ideal gas equation to calculate the volume of 1 mole of an ideal gas at 0°c and. How To Use The Ideal Gas Law Equation.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 How To Use The Ideal Gas Law Equation Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) in such a case, all gases obey an equation of state known as the ideal gas. How To Use The Ideal Gas Law Equation.
From www.youtube.com
LM Unit 9 Intro Ideal Gas Law YouTube How To Use The Ideal Gas Law Equation (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number. in such a case, all gases obey an equation of state known as the ideal gas law: The arrows represent the random. Molecular collisions. How To Use The Ideal Gas Law Equation.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation How To Use The Ideal Gas Law Equation use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) in such a case, all gases obey an equation of state known as the ideal gas law: the. How To Use The Ideal Gas Law Equation.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net How To Use The Ideal Gas Law Equation use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Molecular collisions within a closed container (a propane tank) are shown (right). in such a case, all gases obey an equation of state known as the ideal gas law: the ideal gas law. How To Use The Ideal Gas Law Equation.
From www.slideserve.com
PPT The ideal gas law PowerPoint Presentation, free download ID3608170 How To Use The Ideal Gas Law Equation Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) uses of the ideal gas law including solving for an unknown variable, comparing initial and final. How To Use The Ideal Gas Law Equation.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID How To Use The Ideal Gas Law Equation the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number. in such a case, all gases obey an equation of state known as the ideal gas law: (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) The arrows represent the random. use. How To Use The Ideal Gas Law Equation.
From www.youtube.com
Gas density and PV=nRT, the ideal gas law YouTube How To Use The Ideal Gas Law Equation uses of the ideal gas law including solving for an unknown variable, comparing initial and final states, and finding partial pressure. Molecular collisions within a closed container (a propane tank) are shown (right). we can use the ideal gas equation to calculate the volume of 1 mole of an ideal gas at 0°c and 1 atmosphere pressure. . How To Use The Ideal Gas Law Equation.
From www.youtube.com
Introduction to the Ideal Gas Law Formula YouTube How To Use The Ideal Gas Law Equation use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. we can use the ideal gas equation to calculate the volume of 1 mole of an ideal gas at 0°c and 1 atmosphere pressure. (the universal gas constant is defined as avogadro’s number na. How To Use The Ideal Gas Law Equation.
From worksheetfullsmokers.z22.web.core.windows.net
How To Calculate Gas Law How To Use The Ideal Gas Law Equation in such a case, all gases obey an equation of state known as the ideal gas law: the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant,. How To Use The Ideal Gas Law Equation.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID How To Use The Ideal Gas Law Equation Molecular collisions within a closed container (a propane tank) are shown (right). The arrows represent the random. (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number. use the ideal gas law to calculate. How To Use The Ideal Gas Law Equation.
From www.youtube.com
IDEAL GAS LAW PRACTICE PROBLEMS How to Solve Ideal Gas Law Problems How To Use The Ideal Gas Law Equation use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number. we can use the ideal gas equation to calculate the volume of 1 mole of an. How To Use The Ideal Gas Law Equation.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID How To Use The Ideal Gas Law Equation use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. The arrows represent the random. we can use. How To Use The Ideal Gas Law Equation.
From chemistryguru.com.sg
Ideal Gas Law and Applications How To Use The Ideal Gas Law Equation uses of the ideal gas law including solving for an unknown variable, comparing initial and final states, and finding partial pressure. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. use the ideal gas law to calculate pressure change,. How To Use The Ideal Gas Law Equation.
From mavink.com
Derive Ideal Gas Equation How To Use The Ideal Gas Law Equation we can use the ideal gas equation to calculate the volume of 1 mole of an ideal gas at 0°c and 1 atmosphere pressure. use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Pv = nrt, where n is the number of moles. How To Use The Ideal Gas Law Equation.
From www.chem.fsu.edu
Gas Laws How To Use The Ideal Gas Law Equation the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. use the ideal gas law to calculate pressure change, temperature change,. How To Use The Ideal Gas Law Equation.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID7067134 How To Use The Ideal Gas Law Equation the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number. Molecular collisions within a closed container (a propane tank) are shown (right). use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. uses of the ideal. How To Use The Ideal Gas Law Equation.
From byjus.com
Ideal Gas Law Equation Compressibility Of Natural Gas Chemistry How To Use The Ideal Gas Law Equation we can use the ideal gas equation to calculate the volume of 1 mole of an ideal gas at 0°c and 1 atmosphere pressure. The arrows represent the random. Molecular collisions within a closed container (a propane tank) are shown (right). use the ideal gas equation to solve a problem when the amount of gas is given and. How To Use The Ideal Gas Law Equation.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii How To Use The Ideal Gas Law Equation the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. uses of the ideal gas law including solving for an unknown. How To Use The Ideal Gas Law Equation.
From studylib.net
Ideal Gas Law How To Use The Ideal Gas Law Equation The arrows represent the random. Molecular collisions within a closed container (a propane tank) are shown (right). Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. the ideal gas law is derived from empirical relationships among the pressure, the volume,. How To Use The Ideal Gas Law Equation.
From hdimagegallery.net
Ideal Gas Law Formula For Volume images How To Use The Ideal Gas Law Equation use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) we can use the ideal gas equation to calculate the volume of 1 mole of an ideal gas at. How To Use The Ideal Gas Law Equation.
From materialfullrobin.z13.web.core.windows.net
Gas Laws Equations Sheet How To Use The Ideal Gas Law Equation uses of the ideal gas law including solving for an unknown variable, comparing initial and final states, and finding partial pressure. we can use the ideal gas equation to calculate the volume of 1 mole of an ideal gas at 0°c and 1 atmosphere pressure. the ideal gas law is derived from empirical relationships among the pressure,. How To Use The Ideal Gas Law Equation.
From www.youtube.com
PVM mRT Ideal Gas law 2 YouTube How To Use The Ideal Gas Law Equation (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. in such a case, all gases obey an equation of state known as the ideal gas law: Molecular collisions within a. How To Use The Ideal Gas Law Equation.
From chemistryskills.com
Ideal Gas Law Combined Gas Law Chemistry Skills How To Use The Ideal Gas Law Equation use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. uses of the ideal gas law including solving for an unknown variable, comparing initial and final states, and finding partial pressure. in such a case, all gases obey an equation of state known. How To Use The Ideal Gas Law Equation.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii How To Use The Ideal Gas Law Equation (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant,. How To Use The Ideal Gas Law Equation.
From mmerevise.co.uk
The Ideal Gas Equation MME How To Use The Ideal Gas Law Equation in such a case, all gases obey an equation of state known as the ideal gas law: (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. use. How To Use The Ideal Gas Law Equation.