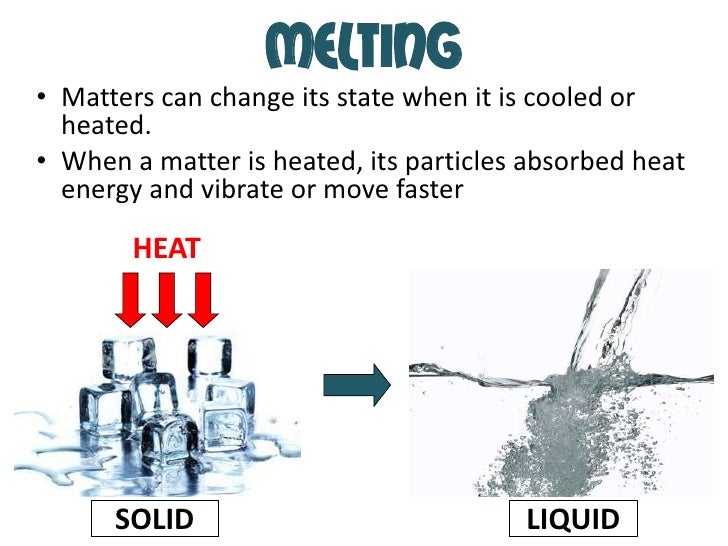
What Happens To The Energy When Ice Melts To Form Liquid . In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. Melting of ice occurs in two steps: In liquids and gases, the transferred heat increases the kinetic energy and thus the speed of the molecules. First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature; This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: The addition of heat energy to a system from its surroundings is an endothermic process. What is the difference between melting and solidification? Continue to apply heat, and the water will turn into water vapour, which is. In other words, ice absorbs energy from the surroundings in order to transition into liquid. What is the difference between boiling and condensation? When heat (a form of energy) is added, the ice melts into liquid water. During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces.
from www.slideshare.net
What is the difference between melting and solidification? What is the difference between boiling and condensation? When heat (a form of energy) is added, the ice melts into liquid water. Continue to apply heat, and the water will turn into water vapour, which is. The addition of heat energy to a system from its surroundings is an endothermic process. Melting of ice occurs in two steps: In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. In other words, ice absorbs energy from the surroundings in order to transition into liquid. In liquids and gases, the transferred heat increases the kinetic energy and thus the speed of the molecules. During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces.
Heat
What Happens To The Energy When Ice Melts To Form Liquid First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature; In other words, ice absorbs energy from the surroundings in order to transition into liquid. The addition of heat energy to a system from its surroundings is an endothermic process. First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature; Continue to apply heat, and the water will turn into water vapour, which is. What is the difference between boiling and condensation? During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. In liquids and gases, the transferred heat increases the kinetic energy and thus the speed of the molecules. What is the difference between melting and solidification? When heat (a form of energy) is added, the ice melts into liquid water. Melting of ice occurs in two steps:
From studybrewmaster.z21.web.core.windows.net
What Happens To A Solid When It Melts What Happens To The Energy When Ice Melts To Form Liquid When heat (a form of energy) is added, the ice melts into liquid water. In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. In other words, ice absorbs energy from the surroundings in order to transition into liquid. During the melting process, the kinetic energy provided through heat enables. What Happens To The Energy When Ice Melts To Form Liquid.
From www.vectorstock.com
Diagram showing how ice melts Royalty Free Vector Image What Happens To The Energy When Ice Melts To Form Liquid In other words, ice absorbs energy from the surroundings in order to transition into liquid. Melting of ice occurs in two steps: What is the difference between boiling and condensation? In liquids and gases, the transferred heat increases the kinetic energy and thus the speed of the molecules. The addition of heat energy to a system from its surroundings is. What Happens To The Energy When Ice Melts To Form Liquid.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, What Happens To The Energy When Ice Melts To Form Liquid This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: What is the difference between boiling and condensation? In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. Melting of ice occurs. What Happens To The Energy When Ice Melts To Form Liquid.
From www.youtube.com
Why is the melting of ice a PHYSICAL change? YouTube What Happens To The Energy When Ice Melts To Form Liquid The addition of heat energy to a system from its surroundings is an endothermic process. What is the difference between melting and solidification? What is the difference between boiling and condensation? Melting of ice occurs in two steps: During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces. Continue to. What Happens To The Energy When Ice Melts To Form Liquid.
From studylib.net
What Happens to Molecules When a Substance Melts? Ice Liquid What Happens To The Energy When Ice Melts To Form Liquid Melting of ice occurs in two steps: Continue to apply heat, and the water will turn into water vapour, which is. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: During the melting process, the kinetic energy provided through heat enables. What Happens To The Energy When Ice Melts To Form Liquid.
From www.slideserve.com
PPT Pascal’s Principle PowerPoint Presentation, free download ID What Happens To The Energy When Ice Melts To Form Liquid What is the difference between boiling and condensation? This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Continue to apply heat, and the water will turn into water vapour, which is. In the case of ice melting under atmospheric pressure, the. What Happens To The Energy When Ice Melts To Form Liquid.
From www.britannica.com
Ice Definition, Structure, Properties, Freezing Point, & Facts What Happens To The Energy When Ice Melts To Form Liquid What is the difference between boiling and condensation? During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces. When heat (a form of energy) is added, the ice melts into liquid water. First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature; Melting of. What Happens To The Energy When Ice Melts To Form Liquid.
From sciencing.com
Does Energy Increase in a Drink When Ice Melts? Sciencing What Happens To The Energy When Ice Melts To Form Liquid In liquids and gases, the transferred heat increases the kinetic energy and thus the speed of the molecules. The addition of heat energy to a system from its surroundings is an endothermic process. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant. What Happens To The Energy When Ice Melts To Form Liquid.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical What Happens To The Energy When Ice Melts To Form Liquid During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: What is the difference between boiling and condensation? First, the phase change. What Happens To The Energy When Ice Melts To Form Liquid.
From quizlet.com
Describe the role of energy when ice melts and when water va Quizlet What Happens To The Energy When Ice Melts To Form Liquid This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. In other words, ice absorbs energy from the surroundings in order to. What Happens To The Energy When Ice Melts To Form Liquid.
From www.britannica.com
phase Definition & Facts Britannica What Happens To The Energy When Ice Melts To Form Liquid Melting of ice occurs in two steps: During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces. What is the difference between melting and solidification? In other words, ice absorbs energy from the surroundings in order to transition into liquid. What is the difference between boiling and condensation? This plot. What Happens To The Energy When Ice Melts To Form Liquid.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical What Happens To The Energy When Ice Melts To Form Liquid In liquids and gases, the transferred heat increases the kinetic energy and thus the speed of the molecules. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: What is the difference between melting and solidification? The addition of heat energy to. What Happens To The Energy When Ice Melts To Form Liquid.
From www.slideshare.net
Heat What Happens To The Energy When Ice Melts To Form Liquid What is the difference between melting and solidification? This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. The addition of heat. What Happens To The Energy When Ice Melts To Form Liquid.
From easyscienceforkids.com
Facts About States of Matter Easy Science For KidsEasy Science For Kids What Happens To The Energy When Ice Melts To Form Liquid This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Melting of ice occurs in two steps: What is the difference between melting and solidification? In liquids and gases, the transferred heat increases the kinetic energy and thus the speed of the. What Happens To The Energy When Ice Melts To Form Liquid.
From news.schoolsdo.org
When ice melts on Earth, it happens layer by layer Voxitatis Blog What Happens To The Energy When Ice Melts To Form Liquid Melting of ice occurs in two steps: What is the difference between melting and solidification? The addition of heat energy to a system from its surroundings is an endothermic process. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: In liquids. What Happens To The Energy When Ice Melts To Form Liquid.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox What Happens To The Energy When Ice Melts To Form Liquid What is the difference between boiling and condensation? Continue to apply heat, and the water will turn into water vapour, which is. In liquids and gases, the transferred heat increases the kinetic energy and thus the speed of the molecules. What is the difference between melting and solidification? In other words, ice absorbs energy from the surroundings in order to. What Happens To The Energy When Ice Melts To Form Liquid.
From www.pinterest.com
The arrangement of water molecules in solid ice, liquid water and water What Happens To The Energy When Ice Melts To Form Liquid In liquids and gases, the transferred heat increases the kinetic energy and thus the speed of the molecules. In other words, ice absorbs energy from the surroundings in order to transition into liquid. Continue to apply heat, and the water will turn into water vapour, which is. First, the phase change occurs and solid (ice) transforms into liquid water at. What Happens To The Energy When Ice Melts To Form Liquid.
From www.youtube.com
What happens to water level, when ice melts। NEET/JEE। Physics।Fluid What Happens To The Energy When Ice Melts To Form Liquid The addition of heat energy to a system from its surroundings is an endothermic process. What is the difference between boiling and condensation? During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces. Melting of ice occurs in two steps: In the case of ice melting under atmospheric pressure, the. What Happens To The Energy When Ice Melts To Form Liquid.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical What Happens To The Energy When Ice Melts To Form Liquid In other words, ice absorbs energy from the surroundings in order to transition into liquid. What is the difference between melting and solidification? First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature; When heat (a form of energy) is added, the ice melts into liquid water. During the melting process, the kinetic energy. What Happens To The Energy When Ice Melts To Form Liquid.
From www.slideserve.com
PPT The Nature of Matter PowerPoint Presentation, free download ID What Happens To The Energy When Ice Melts To Form Liquid What is the difference between boiling and condensation? In other words, ice absorbs energy from the surroundings in order to transition into liquid. What is the difference between melting and solidification? When heat (a form of energy) is added, the ice melts into liquid water. In liquids and gases, the transferred heat increases the kinetic energy and thus the speed. What Happens To The Energy When Ice Melts To Form Liquid.
From www.slideserve.com
PPT What happens when ICE melts? PowerPoint Presentation, free What Happens To The Energy When Ice Melts To Form Liquid In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. When heat (a form of energy) is added, the ice melts into liquid water. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a. What Happens To The Energy When Ice Melts To Form Liquid.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical What Happens To The Energy When Ice Melts To Form Liquid The addition of heat energy to a system from its surroundings is an endothermic process. Continue to apply heat, and the water will turn into water vapour, which is. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: In the case. What Happens To The Energy When Ice Melts To Form Liquid.
From classroom.synonym.com
What Happens When Ice Is Added to Hot Water and How Will the Energy What Happens To The Energy When Ice Melts To Form Liquid First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature; This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: When heat (a form of energy) is added, the ice melts into liquid water. In the. What Happens To The Energy When Ice Melts To Form Liquid.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical What Happens To The Energy When Ice Melts To Form Liquid In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. In other words, ice absorbs energy from the surroundings in order to transition into liquid. The addition of heat energy to a system from its surroundings is an endothermic process. Continue to apply heat, and the water will turn into. What Happens To The Energy When Ice Melts To Form Liquid.
From ar.inspiredpencil.com
Ice Cube Melting In Water What Happens To The Energy When Ice Melts To Form Liquid In other words, ice absorbs energy from the surroundings in order to transition into liquid. During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces. Continue to apply heat, and the water will turn into water vapour, which is. What is the difference between boiling and condensation? First, the phase. What Happens To The Energy When Ice Melts To Form Liquid.
From data.allenai.org
changes of state (lesson 0771) TQA explorer What Happens To The Energy When Ice Melts To Form Liquid When heat (a form of energy) is added, the ice melts into liquid water. In other words, ice absorbs energy from the surroundings in order to transition into liquid. First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature; What is the difference between melting and solidification? What is the difference between boiling and. What Happens To The Energy When Ice Melts To Form Liquid.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical What Happens To The Energy When Ice Melts To Form Liquid During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces. Melting of ice occurs in two steps: In other words, ice absorbs energy from the surroundings in order to transition into liquid. In liquids and gases, the transferred heat increases the kinetic energy and thus the speed of the molecules.. What Happens To The Energy When Ice Melts To Form Liquid.
From apollo.lsc.vsc.edu
Latent Heats sublimation and deposition What Happens To The Energy When Ice Melts To Form Liquid In liquids and gases, the transferred heat increases the kinetic energy and thus the speed of the molecules. Melting of ice occurs in two steps: First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature; What is the difference between boiling and condensation? The addition of heat energy to a system from its surroundings. What Happens To The Energy When Ice Melts To Form Liquid.
From socratic.org
What happens to the energy of its molecules as ice melts into What Happens To The Energy When Ice Melts To Form Liquid In liquids and gases, the transferred heat increases the kinetic energy and thus the speed of the molecules. Continue to apply heat, and the water will turn into water vapour, which is. During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces. What is the difference between boiling and condensation?. What Happens To The Energy When Ice Melts To Form Liquid.
From www.scienceabc.com
What Is The First Law Of Thermodynamics? » ScienceABC What Happens To The Energy When Ice Melts To Form Liquid The addition of heat energy to a system from its surroundings is an endothermic process. In liquids and gases, the transferred heat increases the kinetic energy and thus the speed of the molecules. Melting of ice occurs in two steps: First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature; In other words, ice. What Happens To The Energy When Ice Melts To Form Liquid.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation What Happens To The Energy When Ice Melts To Form Liquid In other words, ice absorbs energy from the surroundings in order to transition into liquid. What is the difference between boiling and condensation? Melting of ice occurs in two steps: The addition of heat energy to a system from its surroundings is an endothermic process. This plot of temperature shows what happens to a 75 g sample of ice initially. What Happens To The Energy When Ice Melts To Form Liquid.
From huntingwaterfalls.com
What Exactly Happens When an Ice Cube Melts? (Simple Science Explained) What Happens To The Energy When Ice Melts To Form Liquid What is the difference between melting and solidification? What is the difference between boiling and condensation? Melting of ice occurs in two steps: First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature; In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. The. What Happens To The Energy When Ice Melts To Form Liquid.
From huntingwaterfalls.com
What Exactly Happens When an Ice Cube Melts? (Simple Science Explained) What Happens To The Energy When Ice Melts To Form Liquid What is the difference between melting and solidification? In liquids and gases, the transferred heat increases the kinetic energy and thus the speed of the molecules. What is the difference between boiling and condensation? First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature; Continue to apply heat, and the water will turn into. What Happens To The Energy When Ice Melts To Form Liquid.
From www.snexplores.org
Explainer What are the different states of matter? What Happens To The Energy When Ice Melts To Form Liquid What is the difference between boiling and condensation? During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: In the case of. What Happens To The Energy When Ice Melts To Form Liquid.
From www.shmoop.com
Chemistry Phase Changes Shmoop Chemistry What Happens To The Energy When Ice Melts To Form Liquid During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces. What is the difference between boiling and condensation? In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. In liquids and gases, the transferred heat increases the kinetic energy and. What Happens To The Energy When Ice Melts To Form Liquid.