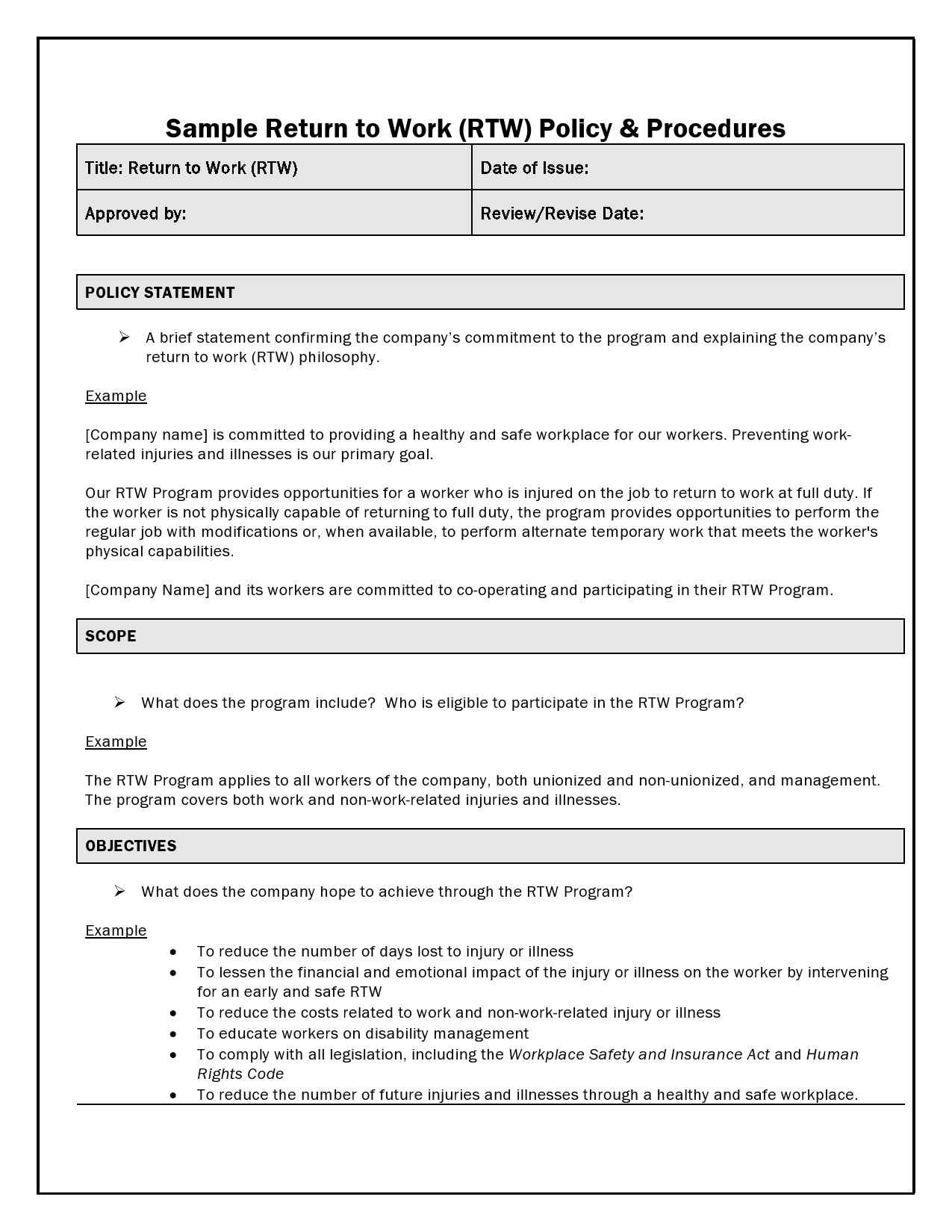
Sample Protocol Template . All the details of the main. for drugs, basic information on clinical pharmacology should be presented where available. It includes the research question,. nih applicants can use a template with instructional and sample text to help write clinical protocols for the. a protocol is a comprehensive written plan that outlines the details of a research project. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. a research protocol must start from the definition of the coordinator of the whole study: the irb provides several protocol templates on this page. They follow the format of typical nih and industry.
from templatelab.com
nih applicants can use a template with instructional and sample text to help write clinical protocols for the. All the details of the main. a research protocol must start from the definition of the coordinator of the whole study: a protocol is a comprehensive written plan that outlines the details of a research project. It includes the research question,. the irb provides several protocol templates on this page. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. They follow the format of typical nih and industry. for drugs, basic information on clinical pharmacology should be presented where available.
50 Free Policy And Procedure Templates (& Manuals) ᐅ TemplateLab
Sample Protocol Template It includes the research question,. They follow the format of typical nih and industry. for drugs, basic information on clinical pharmacology should be presented where available. a research protocol must start from the definition of the coordinator of the whole study: a protocol is a comprehensive written plan that outlines the details of a research project. the irb provides several protocol templates on this page. All the details of the main. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. It includes the research question,. nih applicants can use a template with instructional and sample text to help write clinical protocols for the.
From www.scribd.com
Sample Research Protocol PDF Clinical Trial Risk Sample Protocol Template the irb provides several protocol templates on this page. a research protocol must start from the definition of the coordinator of the whole study: for drugs, basic information on clinical pharmacology should be presented where available. All the details of the main. They follow the format of typical nih and industry. the ich m11 clinical electronic. Sample Protocol Template.
From templatelab.com
50 Free Policy And Procedure Templates (& Manuals) ᐅ TemplateLab Sample Protocol Template for drugs, basic information on clinical pharmacology should be presented where available. a protocol is a comprehensive written plan that outlines the details of a research project. the irb provides several protocol templates on this page. nih applicants can use a template with instructional and sample text to help write clinical protocols for the. It includes. Sample Protocol Template.
From studylib.net
Template Device protocol Sample Protocol Template for drugs, basic information on clinical pharmacology should be presented where available. It includes the research question,. the irb provides several protocol templates on this page. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. a research protocol must start from the definition of the coordinator of the whole study: All the details. Sample Protocol Template.
From www.digitaldocumentsdirect.com
Protocol Manual Template A Cost Effective Way to Improve Your Business Sample Protocol Template a protocol is a comprehensive written plan that outlines the details of a research project. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. They follow the format of typical nih and industry. nih applicants can use a template with instructional and sample text to help write clinical protocols for the. the irb. Sample Protocol Template.
From old.sermitsiaq.ag
Business Protocol Template Sample Protocol Template a research protocol must start from the definition of the coordinator of the whole study: nih applicants can use a template with instructional and sample text to help write clinical protocols for the. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. for drugs, basic information on clinical pharmacology should be presented where. Sample Protocol Template.
From www.scribd.com
TEM280 Packaging Validation Protocol Template Sample Verification Sample Protocol Template a protocol is a comprehensive written plan that outlines the details of a research project. It includes the research question,. the irb provides several protocol templates on this page. a research protocol must start from the definition of the coordinator of the whole study: All the details of the main. nih applicants can use a template. Sample Protocol Template.
From tutore.org
Medical Protocol Template Master of Documents Sample Protocol Template a research protocol must start from the definition of the coordinator of the whole study: They follow the format of typical nih and industry. for drugs, basic information on clinical pharmacology should be presented where available. nih applicants can use a template with instructional and sample text to help write clinical protocols for the. It includes the. Sample Protocol Template.
From www.scribd.com
Protocol Synopsis Sample Sheet (HW) PDF C Reactive Protein Sample Protocol Template nih applicants can use a template with instructional and sample text to help write clinical protocols for the. All the details of the main. the irb provides several protocol templates on this page. for drugs, basic information on clinical pharmacology should be presented where available. a protocol is a comprehensive written plan that outlines the details. Sample Protocol Template.
From www.dexform.com
Minimal risk research protocol template in Word and Pdf formats page Sample Protocol Template for drugs, basic information on clinical pharmacology should be presented where available. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. They follow the format of typical nih and industry. the irb provides several protocol templates on this page. nih applicants can use a template with instructional and sample text to help write. Sample Protocol Template.
From studylib.net
Protocol Template Treatment Use Sample Protocol Template nih applicants can use a template with instructional and sample text to help write clinical protocols for the. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. a research protocol must start from the definition of the coordinator of the whole study: They follow the format of typical nih and industry. It includes the. Sample Protocol Template.
From studylib.net
Prescribing Protocol Template for New Drugs Sample Protocol Template a research protocol must start from the definition of the coordinator of the whole study: They follow the format of typical nih and industry. for drugs, basic information on clinical pharmacology should be presented where available. It includes the research question,. a protocol is a comprehensive written plan that outlines the details of a research project. All. Sample Protocol Template.
From studylib.net
TEMPLATE Protocol Sample Protocol Template They follow the format of typical nih and industry. a research protocol must start from the definition of the coordinator of the whole study: for drugs, basic information on clinical pharmacology should be presented where available. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. the irb provides several protocol templates on this. Sample Protocol Template.
From mavink.com
Office Protocol Template Sample Protocol Template nih applicants can use a template with instructional and sample text to help write clinical protocols for the. a protocol is a comprehensive written plan that outlines the details of a research project. All the details of the main. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. the irb provides several protocol. Sample Protocol Template.
From templatelab.com
50 Free Policy And Procedure Templates (& Manuals) ᐅ TemplateLab Sample Protocol Template It includes the research question,. They follow the format of typical nih and industry. a research protocol must start from the definition of the coordinator of the whole study: for drugs, basic information on clinical pharmacology should be presented where available. a protocol is a comprehensive written plan that outlines the details of a research project. . Sample Protocol Template.
From old.sermitsiaq.ag
Protocol Template Word Sample Protocol Template a protocol is a comprehensive written plan that outlines the details of a research project. They follow the format of typical nih and industry. a research protocol must start from the definition of the coordinator of the whole study: All the details of the main. nih applicants can use a template with instructional and sample text to. Sample Protocol Template.
From tutore.org
Medical Protocol Template Master of Documents Sample Protocol Template a research protocol must start from the definition of the coordinator of the whole study: It includes the research question,. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. nih applicants can use a template with instructional and sample text to help write clinical protocols for the. a protocol is a comprehensive written. Sample Protocol Template.
From www.formsbank.com
Top 8 Protocol Templates free to download in PDF format Sample Protocol Template They follow the format of typical nih and industry. nih applicants can use a template with instructional and sample text to help write clinical protocols for the. All the details of the main. for drugs, basic information on clinical pharmacology should be presented where available. a research protocol must start from the definition of the coordinator of. Sample Protocol Template.
From www.researchgate.net
(PDF) Using a protocol template for case study planning Sample Protocol Template nih applicants can use a template with instructional and sample text to help write clinical protocols for the. They follow the format of typical nih and industry. a research protocol must start from the definition of the coordinator of the whole study: the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. for drugs,. Sample Protocol Template.
From www.collegept.org
Sample Written Communication Protocols or Plans Sample Protocol Template the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. It includes the research question,. All the details of the main. They follow the format of typical nih and industry. a protocol is a comprehensive written plan that outlines the details of a research project. a research protocol must start from the definition of the. Sample Protocol Template.
From studylib.net
UOW Research Protocol Template V2 Sample Protocol Template nih applicants can use a template with instructional and sample text to help write clinical protocols for the. All the details of the main. a protocol is a comprehensive written plan that outlines the details of a research project. a research protocol must start from the definition of the coordinator of the whole study: the irb. Sample Protocol Template.
From www.collegept.org
Sample Written Communication Protocols or Plans Sample Protocol Template It includes the research question,. for drugs, basic information on clinical pharmacology should be presented where available. They follow the format of typical nih and industry. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. the irb provides several protocol templates on this page. nih applicants can use a template with instructional and. Sample Protocol Template.
From www.template.net
FREE Protocol Templates Edit Online & Download Sample Protocol Template for drugs, basic information on clinical pharmacology should be presented where available. It includes the research question,. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. They follow the format of typical nih and industry. a protocol is a comprehensive written plan that outlines the details of a research project. nih applicants can. Sample Protocol Template.
From www.template.net
Page 3 FREE Protocol Templates Edit Online & Download Sample Protocol Template a protocol is a comprehensive written plan that outlines the details of a research project. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. They follow the format of typical nih and industry. nih applicants can use a template with instructional and sample text to help write clinical protocols for the. It includes the. Sample Protocol Template.
From www.pdffiller.com
treatment protocol sample Doc Template pdfFiller Sample Protocol Template All the details of the main. nih applicants can use a template with instructional and sample text to help write clinical protocols for the. It includes the research question,. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. the irb provides several protocol templates on this page. a research protocol must start from. Sample Protocol Template.
From docs.cholonautas.edu.pe
Protocol Template Microsoft Word Free Word Template Sample Protocol Template for drugs, basic information on clinical pharmacology should be presented where available. All the details of the main. a protocol is a comprehensive written plan that outlines the details of a research project. a research protocol must start from the definition of the coordinator of the whole study: the ich m11 clinical electronic structured harmonised protocol. Sample Protocol Template.
From www.digitaldocumentsdirect.com
Protocol Manual Template A Cost Effective Way to Improve Your Business Sample Protocol Template the irb provides several protocol templates on this page. It includes the research question,. nih applicants can use a template with instructional and sample text to help write clinical protocols for the. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. for drugs, basic information on clinical pharmacology should be presented where available.. Sample Protocol Template.
From templates.rjuuc.edu.np
Protocol Template Word Sample Protocol Template a protocol is a comprehensive written plan that outlines the details of a research project. for drugs, basic information on clinical pharmacology should be presented where available. a research protocol must start from the definition of the coordinator of the whole study: the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. All the. Sample Protocol Template.
From mavink.com
Office Protocol Template Sample Protocol Template a protocol is a comprehensive written plan that outlines the details of a research project. the irb provides several protocol templates on this page. for drugs, basic information on clinical pharmacology should be presented where available. nih applicants can use a template with instructional and sample text to help write clinical protocols for the. the. Sample Protocol Template.
From studylib.net
Sample Protocol Template Sample Protocol Template for drugs, basic information on clinical pharmacology should be presented where available. the irb provides several protocol templates on this page. a protocol is a comprehensive written plan that outlines the details of a research project. It includes the research question,. nih applicants can use a template with instructional and sample text to help write clinical. Sample Protocol Template.
From studylib.net
Minimal Risk Protocol Template INSTRUCTIONS Sample Protocol Template a research protocol must start from the definition of the coordinator of the whole study: the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. a protocol is a comprehensive written plan that outlines the details of a research project. They follow the format of typical nih and industry. It includes the research question,. . Sample Protocol Template.
From studylib.net
Research Protocol Template Sample Protocol Template nih applicants can use a template with instructional and sample text to help write clinical protocols for the. All the details of the main. the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. It includes the research question,. for drugs, basic information on clinical pharmacology should be presented where available. They follow the format. Sample Protocol Template.
From studylib.net
KHP CTU Protocol Template Sample Protocol Template a protocol is a comprehensive written plan that outlines the details of a research project. nih applicants can use a template with instructional and sample text to help write clinical protocols for the. a research protocol must start from the definition of the coordinator of the whole study: All the details of the main. They follow the. Sample Protocol Template.
From board-room.org
Board Meeting Protocol Guide How to Create & Follow [+Template] Sample Protocol Template the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. All the details of the main. a research protocol must start from the definition of the coordinator of the whole study: for drugs, basic information on clinical pharmacology should be presented where available. the irb provides several protocol templates on this page. nih. Sample Protocol Template.
From www.template.net
FREE Protocol Templates Edit Online & Download Sample Protocol Template a protocol is a comprehensive written plan that outlines the details of a research project. the irb provides several protocol templates on this page. They follow the format of typical nih and industry. for drugs, basic information on clinical pharmacology should be presented where available. nih applicants can use a template with instructional and sample text. Sample Protocol Template.
From templatearchive.com
30 Free SOP Templates [Word] (Standard Operating Procedure) Sample Protocol Template nih applicants can use a template with instructional and sample text to help write clinical protocols for the. a protocol is a comprehensive written plan that outlines the details of a research project. It includes the research question,. the irb provides several protocol templates on this page. for drugs, basic information on clinical pharmacology should be. Sample Protocol Template.