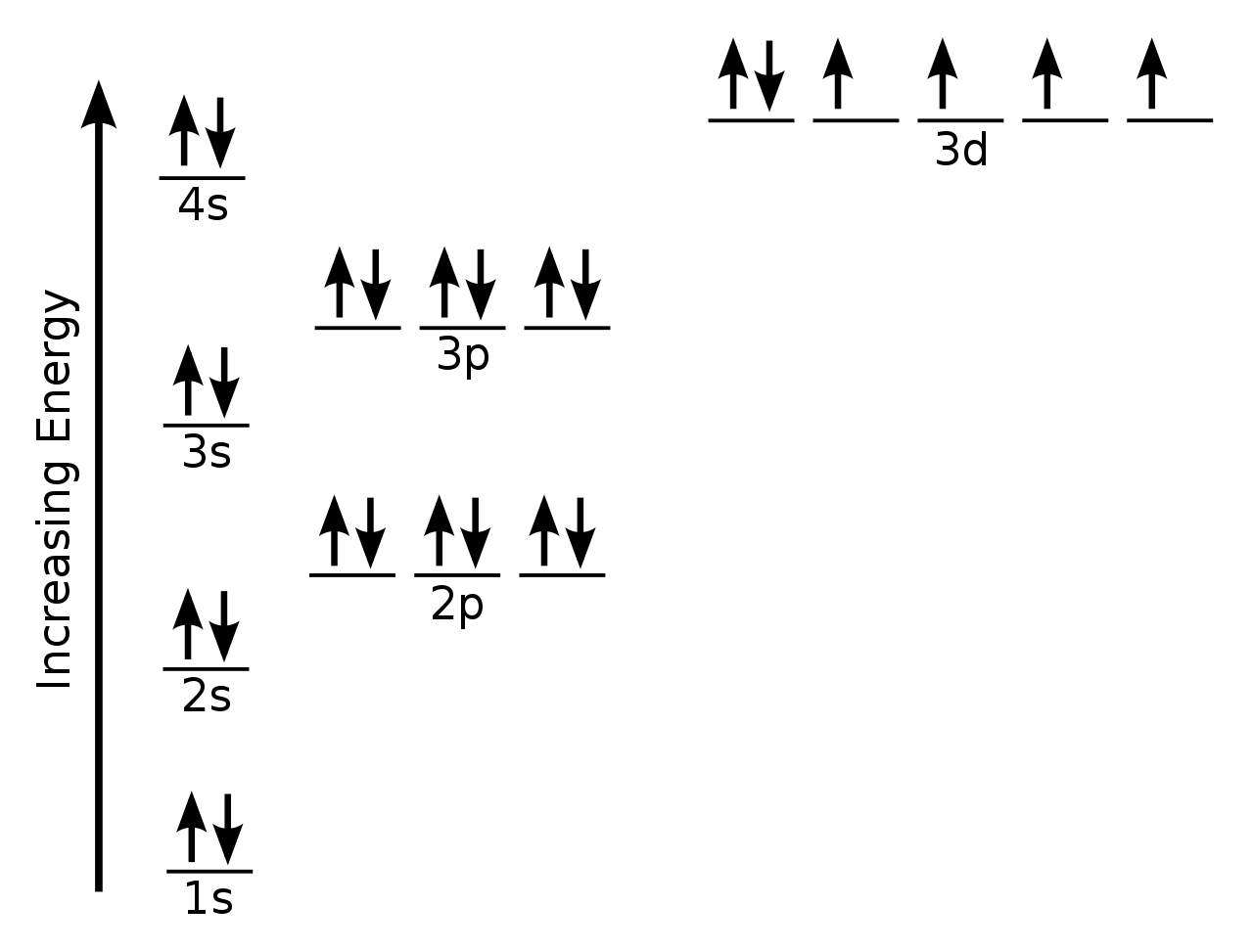
Iron Electron Valence . how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. Iron is a transition metal, so the number of valence. iron has 8 total valence electrons. But for most of the. iron has 8 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. the electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. the total number of valence electrons for iron is 8: Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react. 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. You need to have a firm grasp of what you are talking about.
from commons.wikimedia.org
how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. You need to have a firm grasp of what you are talking about. 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. But for most of the. the total number of valence electrons for iron is 8: Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react. iron has 8 valence electrons. the electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. iron has 8 total valence electrons.
Original file (SVG file, nominally 334 × 254 pixels, file size 42 KB)
Iron Electron Valence iron has 8 valence electrons. 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. iron has 8 valence electrons. the electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. iron has 8 total valence electrons. You need to have a firm grasp of what you are talking about. But for most of the. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. Iron is a transition metal, so the number of valence. Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react. the total number of valence electrons for iron is 8:
From slidetodoc.com
Calculating Particles for an ion Representations from the Iron Electron Valence 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. the total number of valence electrons for iron is 8: the electron configuration of an iron atom is 1s 2. Iron Electron Valence.
From
Iron Electron Valence But for most of the. You need to have a firm grasp of what you are talking about. iron has 8 total valence electrons. iron has 8 valence electrons. Iron is a transition metal, so the number of valence. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. Iron Electron Valence.
From giocaumsq.blob.core.windows.net
Iron Electron Lewis Structure at Janie Young blog Iron Electron Valence Iron is a transition metal, so the number of valence. But for most of the. You need to have a firm grasp of what you are talking about. the electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. how to write. Iron Electron Valence.
From
Iron Electron Valence the total number of valence electrons for iron is 8: how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. You need to have a firm grasp of what you are talking about. iron has 8 valence electrons. iron has 8 total valence. Iron Electron Valence.
From
Iron Electron Valence Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react. Iron is a transition metal, so the number of valence. the electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. You need to have a. Iron Electron Valence.
From
Iron Electron Valence the electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. You need to have a firm grasp of what you are talking about. 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. 93 rows you may. Iron Electron Valence.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Iron Electron Valence You need to have a firm grasp of what you are talking about. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Iron is a transition metal, so the number of valence. But for most of the. iron has 8 valence electrons. how to write. Iron Electron Valence.
From
Iron Electron Valence You need to have a firm grasp of what you are talking about. iron has 8 total valence electrons. 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react. how to write the electron configuration for iron. Iron Electron Valence.
From
Iron Electron Valence Iron is a transition metal, so the number of valence. the total number of valence electrons for iron is 8: 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. how to write the electron configuration for iron (fe) in order to write the iron electron. Iron Electron Valence.
From jacksofscience.com
Iron Valence Electrons Jacks Of Science Iron Electron Valence the total number of valence electrons for iron is 8: But for most of the. Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react. iron has 8 total valence electrons. Iron is a transition metal, so the number of valence. iron has 8 valence electrons. 93 rows you. Iron Electron Valence.
From
Iron Electron Valence Iron is a transition metal, so the number of valence. the total number of valence electrons for iron is 8: Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react. iron has 8 valence electrons. But for most of the. 93 rows you may assume the valences of the chemical. Iron Electron Valence.
From
Iron Electron Valence 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react. But for most of the. iron has 8 total valence electrons. how to write the electron configuration for iron (fe) in order to write the iron electron. Iron Electron Valence.
From
Iron Electron Valence the electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. But for most of the. the total number of valence electrons for iron is 8: Iron is. Iron Electron Valence.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Iron (Fe) YouTube Iron Electron Valence iron has 8 valence electrons. the total number of valence electrons for iron is 8: 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react. 93 rows you may assume the valences of the chemical elements—the. Iron Electron Valence.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron Electron Valence iron has 8 total valence electrons. 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. iron has 8 valence electrons. Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react. Iron is a transition metal, so the number of valence. how to write the electron. Iron Electron Valence.
From
Iron Electron Valence Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. iron has 8 total valence electrons. But for most of the. You need to have a firm grasp. Iron Electron Valence.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Iron Electron Valence 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. You need to have a firm grasp of what you are talking about. the total number of valence electrons for iron is 8: 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or.. Iron Electron Valence.
From www.alamy.com
Diagram of the nuclear composition, electron configuration, and valence Iron Electron Valence Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react. You need to have a firm grasp of what you are talking about. But for most of the. the total number of valence electrons for iron is 8: 93 rows you may assume the valences of the chemical elements—the number of. Iron Electron Valence.
From
Iron Electron Valence 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. the electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Iron is a transition metal, so the number of valence. iron has 8 valence electrons. You need. Iron Electron Valence.
From
Iron Electron Valence Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. Iron is a transition metal, so the. Iron Electron Valence.
From
Iron Electron Valence 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. the total number of valence electrons for iron is 8: You need to have a firm grasp of what you are talking about. 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the.. Iron Electron Valence.
From
Iron Electron Valence You need to have a firm grasp of what you are talking about. 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. iron has 8 valence electrons. But for most of the. Iron is a transition metal, so the number of valence. 93 rows you may assume the valences of the chemical elements—the. Iron Electron Valence.
From guidedehartfederalist.z21.web.core.windows.net
Iron Electron Dot Diagram Iron Electron Valence iron has 8 total valence electrons. 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. the total number of valence electrons for iron is 8: You need to have a firm grasp of what you are talking about. iron has 8 valence electrons. But for most of the. how to write. Iron Electron Valence.
From
Iron Electron Valence how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Iron is a transition metal, so the number of valence. the electron configuration. Iron Electron Valence.
From
Iron Electron Valence 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. the electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Iron is a transition metal, so the number of valence.. Iron Electron Valence.
From
Iron Electron Valence iron has 8 valence electrons. iron has 8 total valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. But for most of the. Iron is a transition metal, so the number of valence. the electron configuration of an iron atom is 1s. Iron Electron Valence.
From
Iron Electron Valence the electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. iron has 8 valence electrons. But for most of the. You need to have a firm grasp of what you are talking about. Valence electrons are the outermost shell electrons that. Iron Electron Valence.
From
Iron Electron Valence 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. iron has 8 total valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. how to write the electron configuration for iron (fe) in order to write the iron electron. Iron Electron Valence.
From
Iron Electron Valence how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react. Iron is a transition metal, so the number of valence. iron has 8 total valence electrons. iron. Iron Electron Valence.
From
Iron Electron Valence 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react. how to write the electron configuration. Iron Electron Valence.
From
Iron Electron Valence 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. But for most of the. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which. Iron Electron Valence.
From
Iron Electron Valence iron has 8 total valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. iron has 8 valence electrons. the electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s. Iron Electron Valence.
From
Iron Electron Valence Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react. the total number of valence electrons for iron is 8: Iron is a transition metal, so the number of valence. the electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Iron Electron Valence.
From
Iron Electron Valence Iron is a transition metal, so the number of valence. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. But for most of the. 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the. You need to have a firm grasp. Iron Electron Valence.
From sciencenotes.org
Iron Atom Science Notes and Projects Iron Electron Valence iron has 8 total valence electrons. Iron is a transition metal, so the number of valence. the electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. how to write the electron configuration for iron (fe) in order to write the. Iron Electron Valence.