Chloride Reaction Formula . Always use the upper case for the first character in the. Acyl chlorides (also known as acid chlorides) are one of a number of. Enter an equation of a chemical reaction and click 'balance'. When hydrogen gas reacts is combined with oxygen gas and the mixture. Chloride can be protonated by strong acids, such as sulfuric acid: \ [\ce {c_7h_ {16} (l) + o_2 (g) → co_2 (g) + h_2o (g) }. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Balance the chemical equation for the combustion of heptane (\ (\ce {c_7h_ {16}}\)). Enter an equation of an ionic chemical equation and press the balance button. This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions, regardless of the source of. Ionic chloride salts react with other salts. The answer will appear below. The balanced equation will be calculated along with the. Nacl + h 2 so 4 → nahso 4 + hcl. Acid chlorides react with ammonia, 1o amines and 2o amines to form amides.
from www.chegg.com
When hydrogen gas reacts is combined with oxygen gas and the mixture. Acyl chlorides (also known as acid chlorides) are one of a number of. Ionic chloride salts react with other salts. Enter an equation of a chemical reaction and click 'balance'. Enter an equation of an ionic chemical equation and press the balance button. The answer will appear below. Nacl + h 2 so 4 → nahso 4 + hcl. Balance the chemical equation for the combustion of heptane (\ (\ce {c_7h_ {16}}\)). Write a balanced chemical equation for the reactions given below: This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions, regardless of the source of.
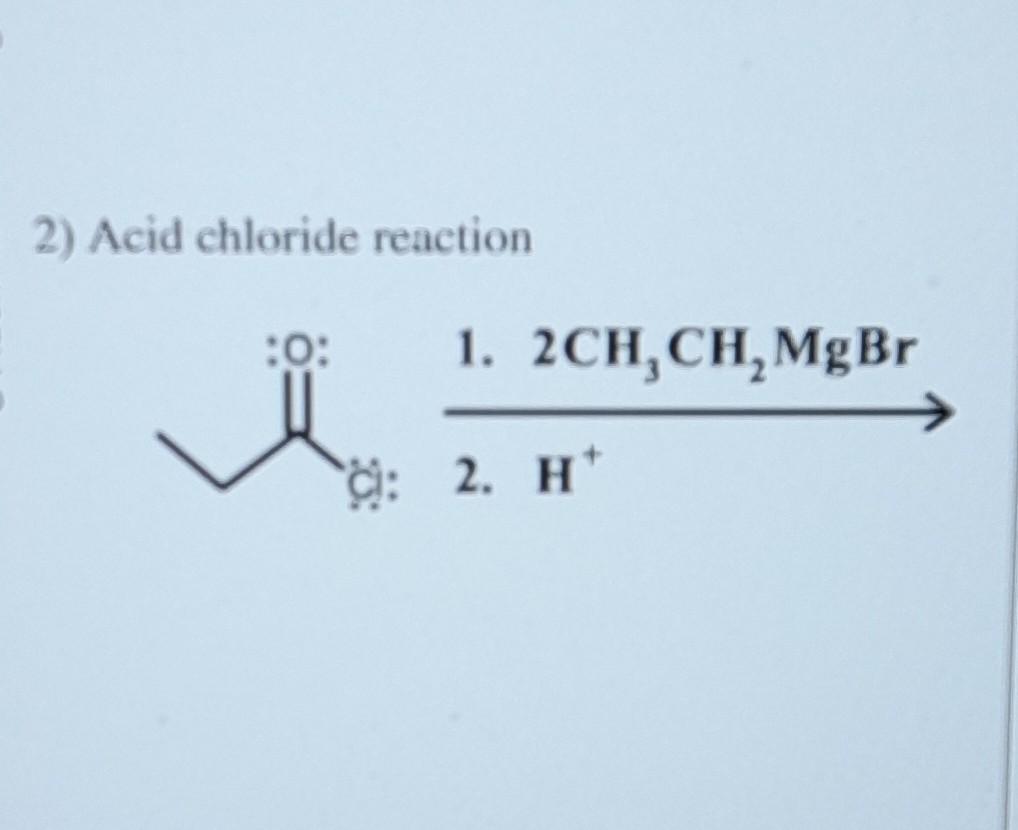
Solved 2) Acid chloride reaction 2. H+ 1. 2CH3CH2MgBr
Chloride Reaction Formula This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions, regardless of the source of. Ionic chloride salts react with other salts. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Enter an equation of a chemical reaction and click 'balance'. Chloride can be protonated by strong acids, such as sulfuric acid: Acyl chlorides (also known as acid chlorides) are one of a number of. The balanced equation will be calculated along with the. Nacl + h 2 so 4 → nahso 4 + hcl. Chemical equation (h2o = h + o) 🛠️ Acid chlorides react with ammonia, 1o amines and 2o amines to form amides. \ [\ce {c_7h_ {16} (l) + o_2 (g) → co_2 (g) + h_2o (g) }. Always use the upper case for the first character in the. Enter an equation of an ionic chemical equation and press the balance button. This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions, regardless of the source of. The answer will appear below. Write a balanced chemical equation for the reactions given below:
From www.chemistrysteps.com
Reactions of Acid Chlorides (ROCl) with Nucleophiles Chemistry Steps Chloride Reaction Formula Chloride can be protonated by strong acids, such as sulfuric acid: Nacl + h 2 so 4 → nahso 4 + hcl. This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions, regardless of the source of. Acid chlorides react with ammonia, 1o amines and 2o amines to form amides. The balanced. Chloride Reaction Formula.
From mungfali.com
Balance The Reaction Of Barium Hydroxide And Ammonium Chloride By The ACF Chloride Reaction Formula The answer will appear below. Enter an equation of an ionic chemical equation and press the balance button. This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions, regardless of the source of. Enter an equation of a chemical reaction and click 'balance'. Use the calculator below to balance chemical equations and. Chloride Reaction Formula.
From ar.inspiredpencil.com
Acid Chloride Functional Group Chloride Reaction Formula When hydrogen gas reacts is combined with oxygen gas and the mixture. The answer will appear below. Ionic chloride salts react with other salts. \ [\ce {c_7h_ {16} (l) + o_2 (g) → co_2 (g) + h_2o (g) }. Chloride can be protonated by strong acids, such as sulfuric acid: Balance the chemical equation for the combustion of heptane (\. Chloride Reaction Formula.
From www.youtube.com
Cobalt Chloride Equilibrium // HSC Chemistry YouTube Chloride Reaction Formula Acid chlorides react with ammonia, 1o amines and 2o amines to form amides. Enter an equation of an ionic chemical equation and press the balance button. Nacl + h 2 so 4 → nahso 4 + hcl. Chemical equation (h2o = h + o) 🛠️ When hydrogen gas reacts is combined with oxygen gas and the mixture. Ionic chloride salts. Chloride Reaction Formula.
From www.youtube.com
NaCl + H2O (Sodium chloride + Water) YouTube Chloride Reaction Formula Balance the chemical equation for the combustion of heptane (\ (\ce {c_7h_ {16}}\)). \ [\ce {c_7h_ {16} (l) + o_2 (g) → co_2 (g) + h_2o (g) }. Acid chlorides react with ammonia, 1o amines and 2o amines to form amides. Enter an equation of an ionic chemical equation and press the balance button. Enter an equation of a chemical. Chloride Reaction Formula.
From chem.libretexts.org
Reactions of Acyl Chlorides with Ammonia Chemistry LibreTexts Chloride Reaction Formula Always use the upper case for the first character in the. Chemical equation (h2o = h + o) 🛠️ Enter an equation of an ionic chemical equation and press the balance button. Enter an equation of a chemical reaction and click 'balance'. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). The balanced equation. Chloride Reaction Formula.
From www.alamy.com
Synthesis reaction sodium chloride formation of sodium metal and Chloride Reaction Formula Enter an equation of a chemical reaction and click 'balance'. The answer will appear below. Nacl + h 2 so 4 → nahso 4 + hcl. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Balance the chemical equation for the combustion of heptane (\ (\ce {c_7h_ {16}}\)). The balanced equation will be calculated. Chloride Reaction Formula.
From www.chemistrysteps.com
Reactions of Acid Chlorides (ROCl) with Nucleophiles Chemistry Steps Chloride Reaction Formula Chloride can be protonated by strong acids, such as sulfuric acid: Chemical equation (h2o = h + o) 🛠️ Acid chlorides react with ammonia, 1o amines and 2o amines to form amides. When hydrogen gas reacts is combined with oxygen gas and the mixture. Acyl chlorides (also known as acid chlorides) are one of a number of. Write a balanced. Chloride Reaction Formula.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Chloride Reaction Formula This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions, regardless of the source of. Chloride can be protonated by strong acids, such as sulfuric acid: Write a balanced chemical equation for the reactions given below: The balanced equation will be calculated along with the. When hydrogen gas reacts is combined with. Chloride Reaction Formula.
From chem.libretexts.org
General reaction mechanism of acid chlorides Chemistry LibreTexts Chloride Reaction Formula Acid chlorides react with ammonia, 1o amines and 2o amines to form amides. This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions, regardless of the source of. The balanced equation will be calculated along with the. Enter an equation of a chemical reaction and click 'balance'. Nacl + h 2 so. Chloride Reaction Formula.
From www.masterorganicchemistry.com
Thionyl Chloride (SOCl2) Master Organic Chemistry Chloride Reaction Formula \ [\ce {c_7h_ {16} (l) + o_2 (g) → co_2 (g) + h_2o (g) }. Nacl + h 2 so 4 → nahso 4 + hcl. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Enter an equation of an ionic chemical equation and press the balance button. Write a balanced chemical equation for. Chloride Reaction Formula.
From www.numerade.com
Consider the reaction between an alcohol and tosyl chloride, followed Chloride Reaction Formula Nacl + h 2 so 4 → nahso 4 + hcl. Enter an equation of a chemical reaction and click 'balance'. Chloride can be protonated by strong acids, such as sulfuric acid: Ionic chloride salts react with other salts. Acid chlorides react with ammonia, 1o amines and 2o amines to form amides. This net ionic equation indicates that solid silver. Chloride Reaction Formula.
From www.youtube.com
Type of Reaction for Na + Cl2 = NaCl (Sodium + Chlorine gas) YouTube Chloride Reaction Formula Enter an equation of a chemical reaction and click 'balance'. Always use the upper case for the first character in the. The balanced equation will be calculated along with the. Ionic chloride salts react with other salts. Write a balanced chemical equation for the reactions given below: Chemical equation (h2o = h + o) 🛠️ Acid chlorides react with ammonia,. Chloride Reaction Formula.
From www.tessshebaylo.com
Chemical Reaction Equation For Ammonium Chloride And Water Tessshebaylo Chloride Reaction Formula Acyl chlorides (also known as acid chlorides) are one of a number of. Chemical equation (h2o = h + o) 🛠️ Enter an equation of a chemical reaction and click 'balance'. Enter an equation of an ionic chemical equation and press the balance button. The answer will appear below. Write a balanced chemical equation for the reactions given below: Balance. Chloride Reaction Formula.
From www.priyamstudycentre.com
Sodium Chloride (NaCl) Uses, Crystal Chloride Reaction Formula This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions, regardless of the source of. The answer will appear below. Acid chlorides react with ammonia, 1o amines and 2o amines to form amides. Nacl + h 2 so 4 → nahso 4 + hcl. Enter an equation of an ionic chemical equation. Chloride Reaction Formula.
From www.onlinemathlearning.com
Chemical Reactions IGCSE Chemistry (solutions, examples, worksheets Chloride Reaction Formula Enter an equation of an ionic chemical equation and press the balance button. \ [\ce {c_7h_ {16} (l) + o_2 (g) → co_2 (g) + h_2o (g) }. Acyl chlorides (also known as acid chlorides) are one of a number of. Balance the chemical equation for the combustion of heptane (\ (\ce {c_7h_ {16}}\)). This net ionic equation indicates that. Chloride Reaction Formula.
From www.youtube.com
How to Balance AlCl3 = Al + Cl2 (Aluminum chloride by Chloride Reaction Formula Balance the chemical equation for the combustion of heptane (\ (\ce {c_7h_ {16}}\)). When hydrogen gas reacts is combined with oxygen gas and the mixture. Always use the upper case for the first character in the. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Chloride can be protonated by strong acids, such as. Chloride Reaction Formula.
From pressbooks.bccampus.ca
6.3 AcidBase Reactions CHEM 1114 Introduction to Chemistry Chloride Reaction Formula When hydrogen gas reacts is combined with oxygen gas and the mixture. Chemical equation (h2o = h + o) 🛠️ The answer will appear below. This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions, regardless of the source of. Enter an equation of an ionic chemical equation and press the balance. Chloride Reaction Formula.
From byjus.com
When does the chloride shift occur? Chloride Reaction Formula Use the calculator below to balance chemical equations and determine the type of reaction (instructions). When hydrogen gas reacts is combined with oxygen gas and the mixture. Acyl chlorides (also known as acid chlorides) are one of a number of. Chloride can be protonated by strong acids, such as sulfuric acid: Chemical equation (h2o = h + o) 🛠️ Ionic. Chloride Reaction Formula.
From www.youtube.com
Equation for Potassium Chloride Dissolving in Water ( KCl + H2O) YouTube Chloride Reaction Formula Chloride can be protonated by strong acids, such as sulfuric acid: Ionic chloride salts react with other salts. Nacl + h 2 so 4 → nahso 4 + hcl. Acid chlorides react with ammonia, 1o amines and 2o amines to form amides. When hydrogen gas reacts is combined with oxygen gas and the mixture. Enter an equation of a chemical. Chloride Reaction Formula.
From www.chegg.com
Solved 2) Acid chloride reaction 2. H+ 1. 2CH3CH2MgBr Chloride Reaction Formula Enter an equation of a chemical reaction and click 'balance'. Chloride can be protonated by strong acids, such as sulfuric acid: Use the calculator below to balance chemical equations and determine the type of reaction (instructions). \ [\ce {c_7h_ {16} (l) + o_2 (g) → co_2 (g) + h_2o (g) }. The answer will appear below. Nacl + h 2. Chloride Reaction Formula.
From www.nagwa.com
Question Video Determining the Chemical Formula of Sodium Chloride Nagwa Chloride Reaction Formula Balance the chemical equation for the combustion of heptane (\ (\ce {c_7h_ {16}}\)). Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Nacl + h 2 so 4 → nahso 4 + hcl. This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions, regardless of the. Chloride Reaction Formula.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Chloride Reaction Formula Enter an equation of a chemical reaction and click 'balance'. The answer will appear below. Write a balanced chemical equation for the reactions given below: Acid chlorides react with ammonia, 1o amines and 2o amines to form amides. Chloride can be protonated by strong acids, such as sulfuric acid: Chemical equation (h2o = h + o) 🛠️ \ [\ce {c_7h_. Chloride Reaction Formula.
From www.chemistrysteps.com
Reactions of Acid Chlorides (ROCl) with Nucleophiles Chemistry Steps Chloride Reaction Formula The answer will appear below. Chemical equation (h2o = h + o) 🛠️ Balance the chemical equation for the combustion of heptane (\ (\ce {c_7h_ {16}}\)). Chloride can be protonated by strong acids, such as sulfuric acid: Write a balanced chemical equation for the reactions given below: Always use the upper case for the first character in the. This net. Chloride Reaction Formula.
From readchemistry.com
Reactions of Alcohols with Thionyl Chloride Read Chemistry Chloride Reaction Formula Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Chemical equation (h2o = h + o) 🛠️ Ionic chloride salts react with other salts. Enter an equation of an ionic chemical equation and press the balance button. The answer will appear below. This net ionic equation indicates that solid silver chloride may be produced. Chloride Reaction Formula.
From byjus.com
The compound formed when aniline reacts with benzene diazonium chloride is Chloride Reaction Formula Nacl + h 2 so 4 → nahso 4 + hcl. Chloride can be protonated by strong acids, such as sulfuric acid: Acyl chlorides (also known as acid chlorides) are one of a number of. Write a balanced chemical equation for the reactions given below: The answer will appear below. \ [\ce {c_7h_ {16} (l) + o_2 (g) → co_2. Chloride Reaction Formula.
From www.chemistrysteps.com
Acyl Chlorides with Grignard and Gilman Reagents Chloride Reaction Formula The answer will appear below. Enter an equation of an ionic chemical equation and press the balance button. Acid chlorides react with ammonia, 1o amines and 2o amines to form amides. Balance the chemical equation for the combustion of heptane (\ (\ce {c_7h_ {16}}\)). This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and. Chloride Reaction Formula.
From www.science-revision.co.uk
Acid halides Chloride Reaction Formula \ [\ce {c_7h_ {16} (l) + o_2 (g) → co_2 (g) + h_2o (g) }. Ionic chloride salts react with other salts. Chloride can be protonated by strong acids, such as sulfuric acid: Balance the chemical equation for the combustion of heptane (\ (\ce {c_7h_ {16}}\)). Nacl + h 2 so 4 → nahso 4 + hcl. Enter an equation. Chloride Reaction Formula.
From openchemistryhelp.blogspot.kr
Chemistry Making acid chlorides from carboxylic acids Chloride Reaction Formula Chemical equation (h2o = h + o) 🛠️ Enter an equation of a chemical reaction and click 'balance'. Enter an equation of an ionic chemical equation and press the balance button. Always use the upper case for the first character in the. Acyl chlorides (also known as acid chlorides) are one of a number of. Chloride can be protonated by. Chloride Reaction Formula.
From www.chemistrysteps.com
Reactions of Acid Chlorides (ROCl) with Nucleophiles Chemistry Steps Chloride Reaction Formula Enter an equation of a chemical reaction and click 'balance'. Chemical equation (h2o = h + o) 🛠️ Always use the upper case for the first character in the. Acid chlorides react with ammonia, 1o amines and 2o amines to form amides. This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. Chloride Reaction Formula.
From www.youtube.com
How to Write the Net Ionic Equation for CoCl2 + NaOH = Co(OH)2 + NaCl Chloride Reaction Formula Chemical equation (h2o = h + o) 🛠️ Ionic chloride salts react with other salts. When hydrogen gas reacts is combined with oxygen gas and the mixture. Acyl chlorides (also known as acid chlorides) are one of a number of. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Enter an equation of a. Chloride Reaction Formula.
From 2012books.lardbucket.org
Reactions in Aqueous Solution Chloride Reaction Formula Chloride can be protonated by strong acids, such as sulfuric acid: \ [\ce {c_7h_ {16} (l) + o_2 (g) → co_2 (g) + h_2o (g) }. Enter an equation of an ionic chemical equation and press the balance button. Nacl + h 2 so 4 → nahso 4 + hcl. Acyl chlorides (also known as acid chlorides) are one of. Chloride Reaction Formula.
From www.youtube.com
Electrolysis of Sodium Chloride Electrochemistry YouTube Chloride Reaction Formula The answer will appear below. Acyl chlorides (also known as acid chlorides) are one of a number of. The balanced equation will be calculated along with the. Chloride can be protonated by strong acids, such as sulfuric acid: Always use the upper case for the first character in the. This net ionic equation indicates that solid silver chloride may be. Chloride Reaction Formula.
From www.essentialchemicalindustry.org
Chlorine Chloride Reaction Formula Acid chlorides react with ammonia, 1o amines and 2o amines to form amides. Enter an equation of an ionic chemical equation and press the balance button. Acyl chlorides (also known as acid chlorides) are one of a number of. Enter an equation of a chemical reaction and click 'balance'. Always use the upper case for the first character in the.. Chloride Reaction Formula.
From www.chemistrysteps.com
Reactions of Acid Chlorides (ROCl) with Nucleophiles Chemistry Steps Chloride Reaction Formula Acyl chlorides (also known as acid chlorides) are one of a number of. The answer will appear below. Chemical equation (h2o = h + o) 🛠️ Nacl + h 2 so 4 → nahso 4 + hcl. Chloride can be protonated by strong acids, such as sulfuric acid: Always use the upper case for the first character in the. Use. Chloride Reaction Formula.