Equivalence Point For Titration Of Strong Base . A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. The equivalence point of a titration. However, in other types of titrations, this is not the case. Suppose 50 ml of 6 m strong acid is added to a base. The titrant and analyte undergo a chemical reaction of known stoichiometry, and so the volume of titrant solution required for complete reaction with the analyte (the equivalence point. Equivalence point (v = 25 ml): Without looking at any graph, a chemist can determine whether or not he has. What is the ph when 49.00 ml of 0.100 m naoh.
from www.numerade.com
Without looking at any graph, a chemist can determine whether or not he has. The titrant and analyte undergo a chemical reaction of known stoichiometry, and so the volume of titrant solution required for complete reaction with the analyte (the equivalence point. However, in other types of titrations, this is not the case. What is the ph when 49.00 ml of 0.100 m naoh. The equivalence point of a titration. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the. Suppose 50 ml of 6 m strong acid is added to a base. Equivalence point (v = 25 ml):
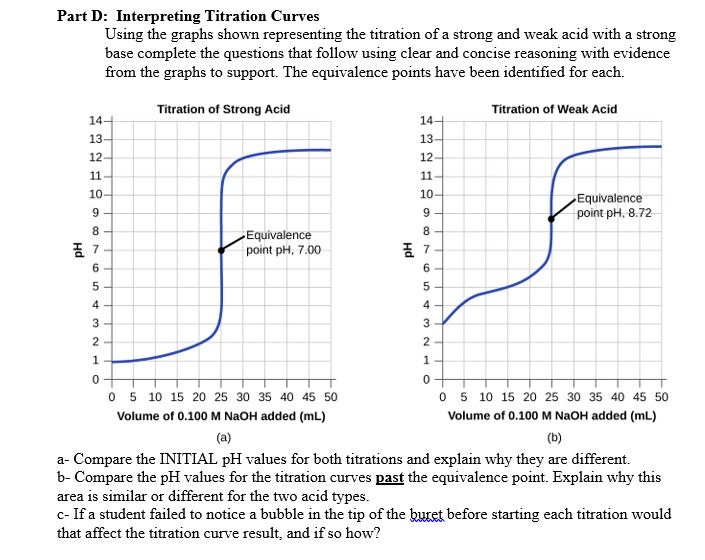
SOLVED Part D Interpreting Titration Curves Using the graphs shown representing the titration
Equivalence Point For Titration Of Strong Base However, in other types of titrations, this is not the case. However, in other types of titrations, this is not the case. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. Suppose 50 ml of 6 m strong acid is added to a base. The titrant and analyte undergo a chemical reaction of known stoichiometry, and so the volume of titrant solution required for complete reaction with the analyte (the equivalence point. Equivalence point (v = 25 ml): Without looking at any graph, a chemist can determine whether or not he has. What is the ph when 49.00 ml of 0.100 m naoh. The equivalence point of a titration. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID5369229 Equivalence Point For Titration Of Strong Base The equivalence point of a titration. Suppose 50 ml of 6 m strong acid is added to a base. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the. Without looking at any graph, a chemist can determine whether or not he has. What is the ph when 49.00 ml of. Equivalence Point For Titration Of Strong Base.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation ID2144898 Equivalence Point For Titration Of Strong Base The titrant and analyte undergo a chemical reaction of known stoichiometry, and so the volume of titrant solution required for complete reaction with the analyte (the equivalence point. The equivalence point of a titration. What is the ph when 49.00 ml of 0.100 m naoh. However, in other types of titrations, this is not the case. Suppose 50 ml of. Equivalence Point For Titration Of Strong Base.
From mungfali.com
Titration Curve Labeled Equivalence Point For Titration Of Strong Base Without looking at any graph, a chemist can determine whether or not he has. The titrant and analyte undergo a chemical reaction of known stoichiometry, and so the volume of titrant solution required for complete reaction with the analyte (the equivalence point. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph. Equivalence Point For Titration Of Strong Base.
From www.youtube.com
Weak acid / strong base titration pH after equivalence point YouTube Equivalence Point For Titration Of Strong Base A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the. Without looking at any graph, a chemist can determine whether or not he has. However, in other types of titrations, this is not the case. For the titration of a strong acid with a strong base, the equivalence point occurs at. Equivalence Point For Titration Of Strong Base.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Equivalence Point For Titration Of Strong Base However, in other types of titrations, this is not the case. The titrant and analyte undergo a chemical reaction of known stoichiometry, and so the volume of titrant solution required for complete reaction with the analyte (the equivalence point. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the. Without looking. Equivalence Point For Titration Of Strong Base.
From www.youtube.com
Conductometric titration of strong acid and weak base (strong acid vs weak base)/Conductometry Equivalence Point For Titration Of Strong Base The equivalence point of a titration. However, in other types of titrations, this is not the case. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the. Suppose 50 ml of 6 m strong acid is added to a base. Equivalence point (v = 25 ml): The titrant and analyte undergo. Equivalence Point For Titration Of Strong Base.
From www.slideserve.com
PPT TITRATION CURVE WEAK ACID WITH STRONG BASE MGKP 2014 PowerPoint Presentation ID2516572 Equivalence Point For Titration Of Strong Base A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the. The titrant and analyte undergo a chemical reaction of known stoichiometry, and so the volume of titrant solution required for complete reaction with the analyte (the equivalence point. What is the ph when 49.00 ml of 0.100 m naoh. Without looking. Equivalence Point For Titration Of Strong Base.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Chemistry JoVe Equivalence Point For Titration Of Strong Base A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the. Equivalence point (v = 25 ml): What is the ph when 49.00 ml of 0.100 m naoh. However, in other types of titrations, this is not the case. Without looking at any graph, a chemist can determine whether or not he. Equivalence Point For Titration Of Strong Base.
From solvedlib.com
The titration curve from the reaction of a strong bas… SolvedLib Equivalence Point For Titration Of Strong Base Suppose 50 ml of 6 m strong acid is added to a base. Equivalence point (v = 25 ml): However, in other types of titrations, this is not the case. The equivalence point of a titration. What is the ph when 49.00 ml of 0.100 m naoh. For the titration of a strong acid with a strong base, the equivalence. Equivalence Point For Titration Of Strong Base.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Equivalence Point For Titration Of Strong Base The equivalence point of a titration. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the. Equivalence point (v = 25 ml): What is the ph when 49.00 ml of 0.100 m naoh. Suppose 50 ml of 6 m strong acid is added to a base. Without looking at any graph,. Equivalence Point For Titration Of Strong Base.
From www.numerade.com
SOLVED Using the following pH curve for the titration of a weak acid with a strong base, if the Equivalence Point For Titration Of Strong Base The equivalence point of a titration. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. Equivalence point (v = 25 ml): Without looking at any graph, a chemist can determine whether or not he has. The. Equivalence Point For Titration Of Strong Base.
From www.numerade.com
SOLVED Part D Interpreting Titration Curves Using the graphs shown representing the titration Equivalence Point For Titration Of Strong Base Suppose 50 ml of 6 m strong acid is added to a base. The titrant and analyte undergo a chemical reaction of known stoichiometry, and so the volume of titrant solution required for complete reaction with the analyte (the equivalence point. The equivalence point of a titration. However, in other types of titrations, this is not the case. What is. Equivalence Point For Titration Of Strong Base.
From www.youtube.com
Titration Weak base/strong acid Before the equivalence point YouTube Equivalence Point For Titration Of Strong Base Suppose 50 ml of 6 m strong acid is added to a base. The titrant and analyte undergo a chemical reaction of known stoichiometry, and so the volume of titrant solution required for complete reaction with the analyte (the equivalence point. What is the ph when 49.00 ml of 0.100 m naoh. Equivalence point (v = 25 ml): A drastic. Equivalence Point For Titration Of Strong Base.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Equivalence Point For Titration Of Strong Base What is the ph when 49.00 ml of 0.100 m naoh. The titrant and analyte undergo a chemical reaction of known stoichiometry, and so the volume of titrant solution required for complete reaction with the analyte (the equivalence point. Without looking at any graph, a chemist can determine whether or not he has. Equivalence point (v = 25 ml): However,. Equivalence Point For Titration Of Strong Base.
From www.writework.com
Titration of amino acids WriteWork Equivalence Point For Titration Of Strong Base Without looking at any graph, a chemist can determine whether or not he has. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. Equivalence point (v = 25 ml): The titrant and analyte undergo a chemical. Equivalence Point For Titration Of Strong Base.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Equivalence Point For Titration Of Strong Base The equivalence point of a titration. The titrant and analyte undergo a chemical reaction of known stoichiometry, and so the volume of titrant solution required for complete reaction with the analyte (the equivalence point. However, in other types of titrations, this is not the case. A drastic rise in ph is observed as the solution composition transitions from acidic to. Equivalence Point For Titration Of Strong Base.
From www.slideshare.net
pH Understanding titration curve Equivalence Point For Titration Of Strong Base The equivalence point of a titration. Equivalence point (v = 25 ml): What is the ph when 49.00 ml of 0.100 m naoh. The titrant and analyte undergo a chemical reaction of known stoichiometry, and so the volume of titrant solution required for complete reaction with the analyte (the equivalence point. For the titration of a strong acid with a. Equivalence Point For Titration Of Strong Base.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Equivalence Point For Titration Of Strong Base For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. What is the ph when 49.00 ml of 0.100 m naoh. A drastic rise in ph is observed as the solution composition transitions from acidic to either. Equivalence Point For Titration Of Strong Base.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Equivalence Point For Titration Of Strong Base Without looking at any graph, a chemist can determine whether or not he has. What is the ph when 49.00 ml of 0.100 m naoh. However, in other types of titrations, this is not the case. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the. Equivalence point (v = 25. Equivalence Point For Titration Of Strong Base.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Equivalence Point For Titration Of Strong Base Suppose 50 ml of 6 m strong acid is added to a base. The equivalence point of a titration. Without looking at any graph, a chemist can determine whether or not he has. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve. Equivalence Point For Titration Of Strong Base.
From www.numerade.com
SOLVED Indicate what is occuning in the titration of = strong base with weak acid by labeling Equivalence Point For Titration Of Strong Base For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. What is the ph when 49.00 ml of 0.100 m naoh. Suppose 50 ml of 6 m strong acid is added to a base. Without looking at. Equivalence Point For Titration Of Strong Base.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Equivalence Point For Titration Of Strong Base Without looking at any graph, a chemist can determine whether or not he has. Equivalence point (v = 25 ml): For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. Suppose 50 ml of 6 m strong. Equivalence Point For Titration Of Strong Base.
From www.youtube.com
Titration Weak base/Strong acid Equivalence Point YouTube Equivalence Point For Titration Of Strong Base However, in other types of titrations, this is not the case. The titrant and analyte undergo a chemical reaction of known stoichiometry, and so the volume of titrant solution required for complete reaction with the analyte (the equivalence point. What is the ph when 49.00 ml of 0.100 m naoh. A drastic rise in ph is observed as the solution. Equivalence Point For Titration Of Strong Base.
From schoolbag.info
Titration and Buffers Acids and Bases Equivalence Point For Titration Of Strong Base A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the. What is the ph when 49.00 ml of 0.100 m naoh. The equivalence point of a titration. Equivalence point (v = 25 ml): Suppose 50 ml of 6 m strong acid is added to a base. Without looking at any graph,. Equivalence Point For Titration Of Strong Base.
From mavink.com
Acid Base Titration Curve Equivalence Point For Titration Of Strong Base Suppose 50 ml of 6 m strong acid is added to a base. Equivalence point (v = 25 ml): However, in other types of titrations, this is not the case. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the. What is the ph when 49.00 ml of 0.100 m naoh.. Equivalence Point For Titration Of Strong Base.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Equivalence Point For Titration Of Strong Base Suppose 50 ml of 6 m strong acid is added to a base. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the. Without looking at any graph, a chemist can determine whether or not he has. The titrant and analyte undergo a chemical reaction of known stoichiometry, and so the. Equivalence Point For Titration Of Strong Base.
From www.youtube.com
Determining the pH During a Strong AcidWeak Base Titration at Equivalence YouTube Equivalence Point For Titration Of Strong Base What is the ph when 49.00 ml of 0.100 m naoh. However, in other types of titrations, this is not the case. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. The titrant and analyte undergo. Equivalence Point For Titration Of Strong Base.
From saylordotorg.github.io
AcidBase Titrations Equivalence Point For Titration Of Strong Base The equivalence point of a titration. However, in other types of titrations, this is not the case. Suppose 50 ml of 6 m strong acid is added to a base. What is the ph when 49.00 ml of 0.100 m naoh. Without looking at any graph, a chemist can determine whether or not he has. The titrant and analyte undergo. Equivalence Point For Titration Of Strong Base.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Equivalence Point For Titration Of Strong Base A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the. However, in other types of titrations, this is not the case. The equivalence point of a titration. What is the ph when 49.00 ml of 0.100 m naoh. The titrant and analyte undergo a chemical reaction of known stoichiometry, and so. Equivalence Point For Titration Of Strong Base.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Equivalence Point For Titration Of Strong Base The equivalence point of a titration. The titrant and analyte undergo a chemical reaction of known stoichiometry, and so the volume of titrant solution required for complete reaction with the analyte (the equivalence point. What is the ph when 49.00 ml of 0.100 m naoh. For the titration of a strong acid with a strong base, the equivalence point occurs. Equivalence Point For Titration Of Strong Base.
From www.youtube.com
Strong acid / strong base titration pH at equivalence point YouTube Equivalence Point For Titration Of Strong Base What is the ph when 49.00 ml of 0.100 m naoh. However, in other types of titrations, this is not the case. Suppose 50 ml of 6 m strong acid is added to a base. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the. Equivalence Point For Titration Of Strong Base.
From philschatz.com
AcidBase Titrations · Chemistry Equivalence Point For Titration Of Strong Base The equivalence point of a titration. Suppose 50 ml of 6 m strong acid is added to a base. Equivalence point (v = 25 ml): However, in other types of titrations, this is not the case. Without looking at any graph, a chemist can determine whether or not he has. The titrant and analyte undergo a chemical reaction of known. Equivalence Point For Titration Of Strong Base.
From www.slideserve.com
PPT Titration of Strong Monoprotic Acid and Base PowerPoint Presentation ID2321643 Equivalence Point For Titration Of Strong Base Without looking at any graph, a chemist can determine whether or not he has. What is the ph when 49.00 ml of 0.100 m naoh. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. Equivalence point. Equivalence Point For Titration Of Strong Base.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Equivalence Point For Titration Of Strong Base For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. Without looking at any graph, a chemist can determine whether or not he has. The equivalence point of a titration. A drastic rise in ph is observed. Equivalence Point For Titration Of Strong Base.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID5369229 Equivalence Point For Titration Of Strong Base For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. Suppose 50 ml of 6 m strong acid is added to a base. However, in other types of titrations, this is not the case. The equivalence point. Equivalence Point For Titration Of Strong Base.