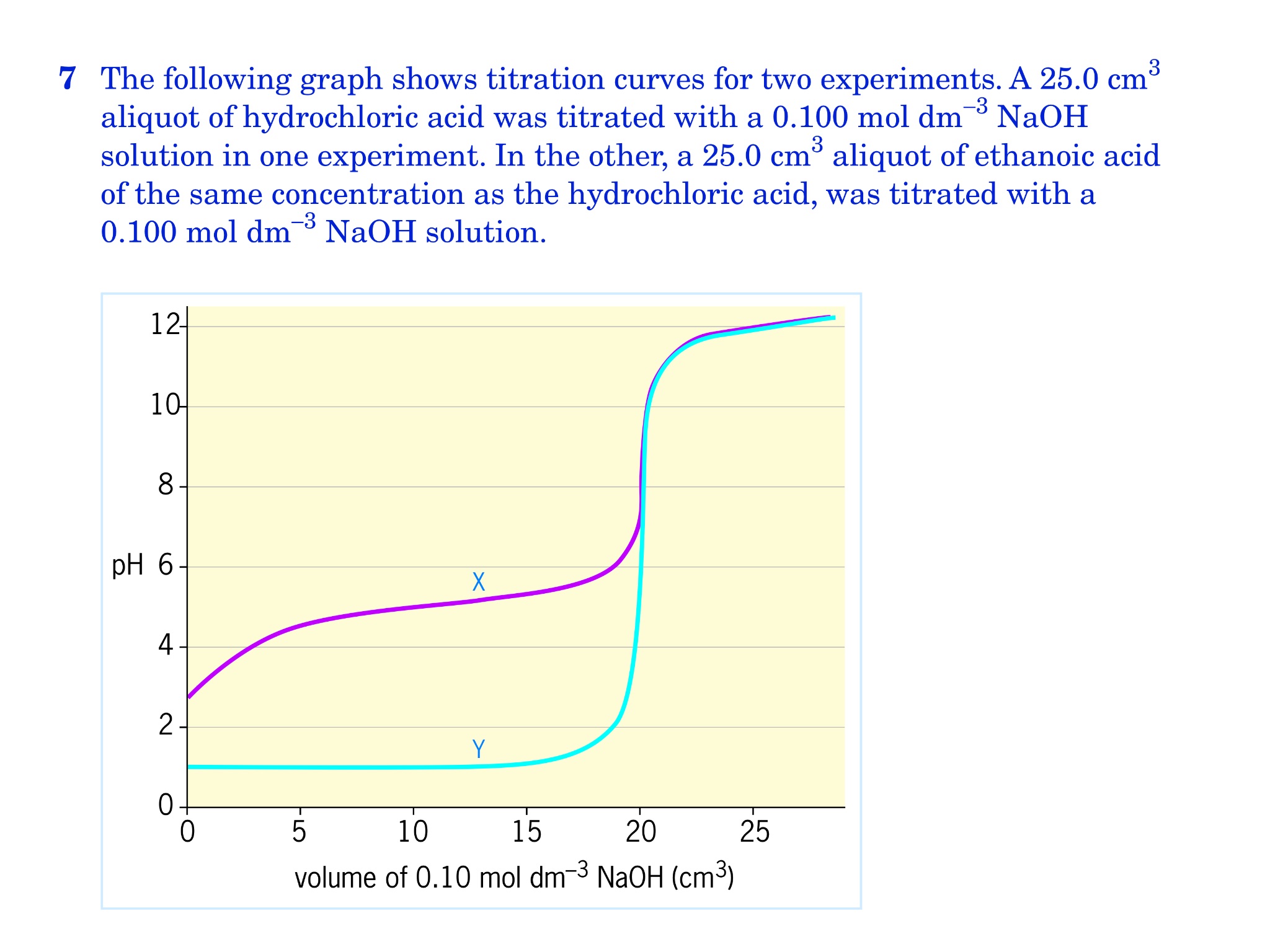
How To Calculate The Concentration Of A Solution In Titration . Calculate the concentration of the hydrochloric acid solution. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. This video walks through common examples of calculation problems for titration. The former quantity could be obtained via a. How to do titration calculations. A titration experiment aims to determine the concentration of an unknown. The process of calculating concentration from titration data is described and illustrated. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. M av a = m bv b. Calculate the amount of sodium hydroxide in moles.
from socratic.org
M av a = m bv b. Calculate the concentration of the hydrochloric acid solution. Calculate the amount of sodium hydroxide in moles. A titration experiment aims to determine the concentration of an unknown. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. How to do titration calculations. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. The former quantity could be obtained via a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
How to calculate the concentration of the acid solutions? Socratic
How To Calculate The Concentration Of A Solution In Titration This video walks through common examples of calculation problems for titration. A titration experiment aims to determine the concentration of an unknown. How to do titration calculations. Calculate the concentration of the hydrochloric acid solution. The process of calculating concentration from titration data is described and illustrated. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The former quantity could be obtained via a. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. Calculate the amount of sodium hydroxide in moles. M av a = m bv b. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. This video walks through common examples of calculation problems for titration.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube How To Calculate The Concentration Of A Solution In Titration Calculate the amount of sodium hydroxide in moles. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. Calculate the concentration of the hydrochloric acid solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration experiment aims to determine the. How To Calculate The Concentration Of A Solution In Titration.
From www.numerade.com
SOLVEDCalculate the concentration (in molarity) of an mathrm{NaOH} solution if 25.0 mathrm How To Calculate The Concentration Of A Solution In Titration M av a = m bv b. How to do titration calculations. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. A titration experiment aims to determine the concentration of an unknown. A. How To Calculate The Concentration Of A Solution In Titration.
From www.chegg.com
Solved TITRATION ⋅ STANDARDIZATION OF SODIUM HYDROXIDE How To Calculate The Concentration Of A Solution In Titration The process of calculating concentration from titration data is described and illustrated. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. A titration experiment aims to determine the concentration of an unknown. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong. How To Calculate The Concentration Of A Solution In Titration.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Calculate The Concentration Of A Solution In Titration A titration experiment aims to determine the concentration of an unknown. M av a = m bv b. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. Calculate the amount of sodium hydroxide in moles. How to do titration calculations. Calculate the concentration of the hydrochloric acid solution. A titration is a. How To Calculate The Concentration Of A Solution In Titration.
From www.youtube.com
The titration of 25 0 mL of an unknown concentration of H2SO4 solution requires 83 6 mL of 0 12 How To Calculate The Concentration Of A Solution In Titration The process of calculating concentration from titration data is described and illustrated. M av a = m bv b. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. The former quantity could be obtained via a. Calculate the concentration of the hydrochloric acid solution. This video walks through common examples of calculation. How To Calculate The Concentration Of A Solution In Titration.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube How To Calculate The Concentration Of A Solution In Titration Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. A titration experiment aims to determine the concentration of an unknown. This video walks through common examples of calculation problems for titration. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved.. How To Calculate The Concentration Of A Solution In Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download ID3199214 How To Calculate The Concentration Of A Solution In Titration Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. The process of calculating concentration from titration data is described and illustrated. The former quantity could be obtained via a. Calculate the concentration of the hydrochloric acid solution. This video walks through common examples of calculation problems for titration. Calculate the amount of. How To Calculate The Concentration Of A Solution In Titration.
From www.youtube.com
[Example] How to Find the Concentration of a Solution. YouTube How To Calculate The Concentration Of A Solution In Titration Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. M av a = m bv b. How to do titration calculations. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution. How To Calculate The Concentration Of A Solution In Titration.
From quizzschooldrenching.z14.web.core.windows.net
How To Find Concentration Of Solutions How To Calculate The Concentration Of A Solution In Titration A titration experiment aims to determine the concentration of an unknown. How to do titration calculations. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. The process of calculating concentration from titration data is described and illustrated. Calculate the amount of sodium hydroxide in moles. Calculate the concentration of the hydrochloric acid solution. To. How To Calculate The Concentration Of A Solution In Titration.
From www.youtube.com
AcidBase Titrations Calculating Concentration of a Standard Solution YouTube How To Calculate The Concentration Of A Solution In Titration A titration experiment aims to determine the concentration of an unknown. M av a = m bv b. Calculate the concentration of the hydrochloric acid solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. This video walks through common examples of calculation problems for titration. Titration is a. How To Calculate The Concentration Of A Solution In Titration.
From socratic.org
How to calculate the concentration of the acid solutions? Socratic How To Calculate The Concentration Of A Solution In Titration Calculate the amount of sodium hydroxide in moles. M av a = m bv b. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. A titration experiment aims to determine the concentration of an unknown. How to do titration calculations. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with. How To Calculate The Concentration Of A Solution In Titration.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Foundation How To Calculate The Concentration Of A Solution In Titration Calculate the amount of sodium hydroxide in moles. M av a = m bv b. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. The process of calculating concentration from titration data is described and illustrated. The former quantity could be obtained via a. Let's assume you are. How To Calculate The Concentration Of A Solution In Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download ID3199214 How To Calculate The Concentration Of A Solution In Titration The former quantity could be obtained via a. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is. How To Calculate The Concentration Of A Solution In Titration.
From chem.libretexts.org
4.3 Solution Concentrations Chemistry LibreTexts How To Calculate The Concentration Of A Solution In Titration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. A titration experiment aims to determine the concentration of an unknown. The former quantity could be obtained via a. Titration is a method. How To Calculate The Concentration Of A Solution In Titration.
From www.slideserve.com
PPT Concentration of Solutions and the Concentration/Volume Relationship PowerPoint How To Calculate The Concentration Of A Solution In Titration The process of calculating concentration from titration data is described and illustrated. This video walks through common examples of calculation problems for titration. M av a = m bv b. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. Calculate the amount of sodium hydroxide in moles. To calculate concentration, we need to know. How To Calculate The Concentration Of A Solution In Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download ID3199214 How To Calculate The Concentration Of A Solution In Titration A titration experiment aims to determine the concentration of an unknown. Calculate the concentration of the hydrochloric acid solution. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. The process of calculating concentration from titration data is described and illustrated. M av a = m bv b. A. How To Calculate The Concentration Of A Solution In Titration.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation ID4401864 How To Calculate The Concentration Of A Solution In Titration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. The process of calculating concentration from titration data is described and illustrated. M av a = m bv b.. How To Calculate The Concentration Of A Solution In Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download ID3199214 How To Calculate The Concentration Of A Solution In Titration To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. How to do titration calculations. Calculate the concentration of the hydrochloric acid solution. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. M av a = m bv b. Let's assume you are. How To Calculate The Concentration Of A Solution In Titration.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Calculate The Concentration Of A Solution In Titration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Calculate the amount of sodium hydroxide in moles. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. The process of calculating concentration from titration data is described and illustrated. The former quantity could be. How To Calculate The Concentration Of A Solution In Titration.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration Khan Academy YouTube How To Calculate The Concentration Of A Solution In Titration Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. Calculate the amount of sodium hydroxide in moles. A titration experiment aims to determine the concentration of an unknown. Calculate the concentration of the hydrochloric acid solution. To calculate concentration, we need to know the amount of naoh and the volume of solution in which. How To Calculate The Concentration Of A Solution In Titration.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation How To Calculate The Concentration Of A Solution In Titration Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. M av a = m bv b. The process of calculating concentration from titration data is described and illustrated. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. This video walks through. How To Calculate The Concentration Of A Solution In Titration.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Calculate The Concentration Of A Solution In Titration The former quantity could be obtained via a. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. A titration experiment aims to determine the concentration of an unknown. Calculate the amount of sodium hydroxide in moles. How to do titration calculations. A titration is a laboratory technique used to precisely measure molar. How To Calculate The Concentration Of A Solution In Titration.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp How To Calculate The Concentration Of A Solution In Titration Calculate the concentration of the hydrochloric acid solution. A titration experiment aims to determine the concentration of an unknown. The process of calculating concentration from titration data is described and illustrated. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Let's assume you are titrating a strong acid (10. How To Calculate The Concentration Of A Solution In Titration.
From www.youtube.com
Calculate Concentration (Example) YouTube How To Calculate The Concentration Of A Solution In Titration Calculate the amount of sodium hydroxide in moles. How to do titration calculations. This video walks through common examples of calculation problems for titration. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. A titration experiment aims to determine the concentration of an unknown. M av a = m bv b. Calculate. How To Calculate The Concentration Of A Solution In Titration.
From quizmischances.z4.web.core.windows.net
How To Calculate Final Concentration How To Calculate The Concentration Of A Solution In Titration To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. Calculate the concentration of the hydrochloric acid solution. A titration experiment aims to determine the concentration of an unknown. Titration is a. How To Calculate The Concentration Of A Solution In Titration.
From giorlbtcj.blob.core.windows.net
How To Calculate Molar Concentration In Titration at Tessie Donato blog How To Calculate The Concentration Of A Solution In Titration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. This video walks through common examples of calculation problems for titration. How to do titration calculations. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. A titration experiment aims to determine the. How To Calculate The Concentration Of A Solution In Titration.
From wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Calculate The Concentration Of A Solution In Titration How to do titration calculations. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Calculate the amount of sodium hydroxide in moles. The former quantity could be obtained via a. A titration experiment aims to determine the concentration of an unknown. Let's assume you are titrating a strong acid. How To Calculate The Concentration Of A Solution In Titration.
From azwikihow.blogspot.com
PREPARATION OF STANDARD SOLUTIONS (TITRATION) AZ WIKI HOW How To Calculate The Concentration Of A Solution In Titration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Calculate the amount of sodium hydroxide in moles. The former quantity could be obtained via a. A titration experiment aims to determine the concentration of an unknown. To calculate concentration, we need to know the amount of naoh and the. How To Calculate The Concentration Of A Solution In Titration.
From www.chegg.com
Solved To calculate the concentration of a solution using How To Calculate The Concentration Of A Solution In Titration This video walks through common examples of calculation problems for titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The process of calculating concentration from titration data is described and illustrated. Calculate the amount of sodium hydroxide in moles. A titration experiment aims to determine the concentration of. How To Calculate The Concentration Of A Solution In Titration.
From www.youtube.com
1.3 Concentration of solutions YouTube How To Calculate The Concentration Of A Solution In Titration How to do titration calculations. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. The process of calculating concentration from titration data is described and illustrated. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. A titration is a laboratory technique used to precisely measure molar. How To Calculate The Concentration Of A Solution In Titration.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Calculate The Concentration Of A Solution In Titration The former quantity could be obtained via a. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. This video walks through common examples of calculation problems for titration. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. M av a = m bv b. How to. How To Calculate The Concentration Of A Solution In Titration.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations, Examples, Solution Stoichiometry How To Calculate The Concentration Of A Solution In Titration M av a = m bv b. The process of calculating concentration from titration data is described and illustrated. The former quantity could be obtained via a. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. Let's assume you are titrating a strong acid (10 ml unknown concentration. How To Calculate The Concentration Of A Solution In Titration.
From www.youtube.com
Calculating Ion Concentrations in Solution YouTube How To Calculate The Concentration Of A Solution In Titration Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. M av a = m bv b. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. This video walks through common examples of calculation problems for titration. A titration experiment aims to determine the concentration of an. How To Calculate The Concentration Of A Solution In Titration.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Calculate The Concentration Of A Solution In Titration This video walks through common examples of calculation problems for titration. The process of calculating concentration from titration data is described and illustrated. Calculate the concentration of the hydrochloric acid solution. M av a = m bv b. The former quantity could be obtained via a. A titration is a laboratory technique used to precisely measure molar concentration of an. How To Calculate The Concentration Of A Solution In Titration.
From theedge.com.hk
Chemistry How To Titration The Edge How To Calculate The Concentration Of A Solution In Titration A titration experiment aims to determine the concentration of an unknown. Calculate the amount of sodium hydroxide in moles. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The process of calculating concentration from titration data is described and illustrated. Let's assume you are titrating a strong acid (10. How To Calculate The Concentration Of A Solution In Titration.