Aluminum Magnesium Hydroxide Chemical Formula . Predict which forms an anion, which forms a cation, and the charges of each ion. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. Magnesium and nitrogen react to form an ionic compound. Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer.
from www.chemicalslearning.com
Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. Predict which forms an anion, which forms a cation, and the charges of each ion. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer. Magnesium and nitrogen react to form an ionic compound.
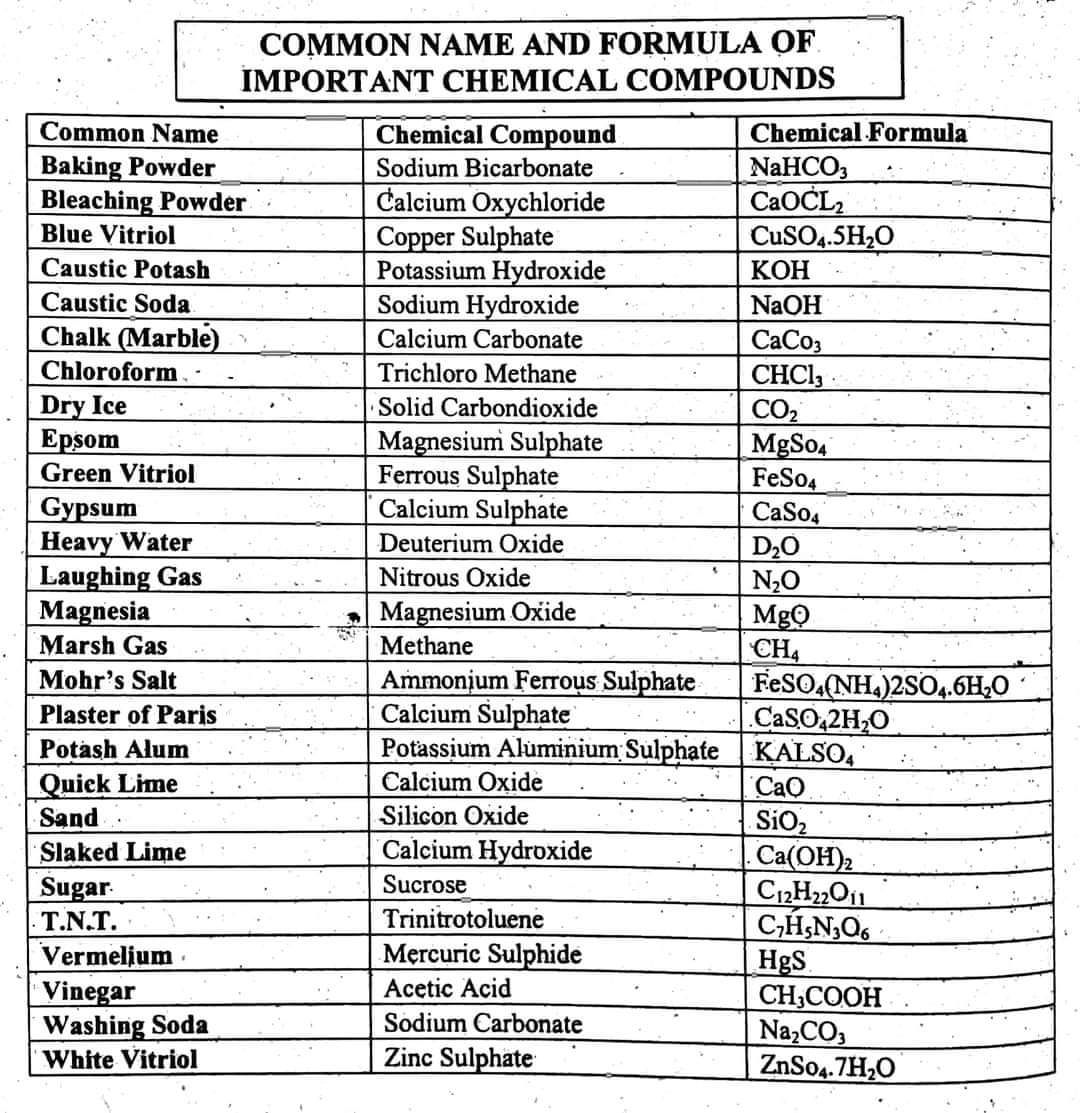
Formula of Important Chemical Compounds
Aluminum Magnesium Hydroxide Chemical Formula Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer. Predict which forms an anion, which forms a cation, and the charges of each ion. Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer. Magnesium and nitrogen react to form an ionic compound. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of.
From pharmog.com
Aluminum oxide + Magnesium hydroxide + Oxethazaine chemical Pharmog Aluminum Magnesium Hydroxide Chemical Formula Magnesium and nitrogen react to form an ionic compound. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. When an ionic compound is formed from. Aluminum Magnesium Hydroxide Chemical Formula.
From testbook.com
Magnesium Hydroxide Definition, Symbol, Examples, Structure Aluminum Magnesium Hydroxide Chemical Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Predict which forms an anion, which forms a cation, and the charges of each ion.. Aluminum Magnesium Hydroxide Chemical Formula.
From lab.honeywell.com
Magnesium hydroxide 310093 Honeywell Research Chemicals Aluminum Magnesium Hydroxide Chemical Formula Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. Predict which forms an anion, which forms a cation, and the charges of each ion. Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer. The. Aluminum Magnesium Hydroxide Chemical Formula.
From animalia-life.club
Magnesium Hydroxide Formula Aluminum Magnesium Hydroxide Chemical Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as. Aluminum Magnesium Hydroxide Chemical Formula.
From dinosenglish.edu.vn
Álbumes 95+ Foto Estructura Molecular Del Oxido De Magnesio Mirada Tensa Aluminum Magnesium Hydroxide Chemical Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three. Aluminum Magnesium Hydroxide Chemical Formula.
From www.glentham.com
Aluminium hydroxide, technical (CAS 21645512) Glentham Life Sciences Aluminum Magnesium Hydroxide Chemical Formula When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Magnesium and nitrogen react to form an ionic compound. Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. Aluminium hydroxide, al (oh)3,. Aluminum Magnesium Hydroxide Chemical Formula.
From www.guidechem.com
125514694 Aluminum magnesium hydroxide sulfate (Al5Mg10(OH)31(SO4)2) Guidechem Aluminum Magnesium Hydroxide Chemical Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as. Aluminum Magnesium Hydroxide Chemical Formula.
From www.chemicalslearning.com
Formula of Important Chemical Compounds Aluminum Magnesium Hydroxide Chemical Formula Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and. Aluminum Magnesium Hydroxide Chemical Formula.
From integratedlaboratories.in
Aluminium Magnesium hydroxide (Intecid) Integrated laboratories Pvt. Ltd. Aluminum Magnesium Hydroxide Chemical Formula Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also. Aluminum Magnesium Hydroxide Chemical Formula.
From www.drugs.com
MagAL Liquid (Pharmaceutical Associates, Inc.) Aluminum Hydroxide 200mg in 5mL, Magnesium Aluminum Magnesium Hydroxide Chemical Formula Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. Aluminum Magnesium Hydroxide Chemical Formula.
From ar.inspiredpencil.com
Magnesium Hydroxide Structure Aluminum Magnesium Hydroxide Chemical Formula Magnesium and nitrogen react to form an ionic compound. Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. Predict which forms an anion, which forms a cation, and the charges of each ion. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. Aluminum Magnesium Hydroxide Chemical Formula.
From www.indiamart.com
Aluminum Magnesium Hydroxide at Rs 110/kg Magnesium Hydroxide in Valsad ID 2850484951688 Aluminum Magnesium Hydroxide Chemical Formula Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. Aluminum Magnesium Hydroxide Chemical Formula.
From lilynewsbranch.blogspot.com
Which Chemical Equation Shows the Dissociation of Magnesium Hydroxide Aluminum Magnesium Hydroxide Chemical Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and. Aluminum Magnesium Hydroxide Chemical Formula.
From www.dreamstime.com
Magnesium Hydroxide Molecular Structure, 3d Model Molecule, Food Additive E528, Structural Aluminum Magnesium Hydroxide Chemical Formula Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The lighter group 3a metals (aluminum, galium and indium), along with scandium. Aluminum Magnesium Hydroxide Chemical Formula.
From www.dreamstime.com
Magnesium Hydroxide Molecule 3d Rendering, Flat Molecular Structure with Chemical Formula and Aluminum Magnesium Hydroxide Chemical Formula Predict which forms an anion, which forms a cation, and the charges of each ion. Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−.. Aluminum Magnesium Hydroxide Chemical Formula.
From www.bambangpharma.com
Aluminum Hydroxide + Magnesium Hydroxide (Zilgam) 200mg/200mg Chewable Bambang Pharmaceutical Aluminum Magnesium Hydroxide Chemical Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+. Aluminum Magnesium Hydroxide Chemical Formula.
From www.youtube.com
How to write the formula for magnesium hydroxide YouTube Aluminum Magnesium Hydroxide Chemical Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Predict which forms an anion, which forms a cation, and the charges of each ion. Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. Aluminium. Aluminum Magnesium Hydroxide Chemical Formula.
From animalia-life.club
Magnesium Hydroxide Formula Aluminum Magnesium Hydroxide Chemical Formula When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer. Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three. Aluminum Magnesium Hydroxide Chemical Formula.
From infinitylearn.com
Magnesium hydroxide Formula Infinity Learn Aluminum Magnesium Hydroxide Chemical Formula Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer. Predict which forms an anion, which forms a cation, and the charges of each ion. The. Aluminum Magnesium Hydroxide Chemical Formula.
From www.sarthaks.com
Write down the formulae of these compounds, using criss cross method? i) Sodium oxide ii Aluminum Magnesium Hydroxide Chemical Formula Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also. Aluminum Magnesium Hydroxide Chemical Formula.
From fda.report
ANTACID aluminum hydroxide, magnesium hydroxide, simethicone liquid Aluminum Magnesium Hydroxide Chemical Formula When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Predict which forms an anion, which forms a cation, and the charges of each ion.. Aluminum Magnesium Hydroxide Chemical Formula.
From brainly.in
1.write the molecular formula for the following by criss cross method a. Magnesium hydroxide b Aluminum Magnesium Hydroxide Chemical Formula When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Magnesium and nitrogen react to form an ionic compound. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Mg + al(oh)3 = mg(oh)2 +. Aluminum Magnesium Hydroxide Chemical Formula.
From animalia-life.club
Magnesium Hydroxide Formula Aluminum Magnesium Hydroxide Chemical Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three. Aluminum Magnesium Hydroxide Chemical Formula.
From aluminumhydroxidesaidan.blogspot.com
Aluminum Hydroxide Magnesium Hydroxide Aluminum Hydroxide Simethicone Suspension Aluminum Magnesium Hydroxide Chemical Formula Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer. Magnesium and nitrogen react to form an ionic compound. The lighter group 3a metals (aluminum, galium. Aluminum Magnesium Hydroxide Chemical Formula.
From www.youtube.com
How to Write the Formula for Magnesium hydroxide YouTube Aluminum Magnesium Hydroxide Chemical Formula Magnesium and nitrogen react to form an ionic compound. Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer. Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. The lighter group 3a metals (aluminum, galium. Aluminum Magnesium Hydroxide Chemical Formula.
From ar.inspiredpencil.com
Magnesium Hydroxide Structure Aluminum Magnesium Hydroxide Chemical Formula Magnesium and nitrogen react to form an ionic compound. Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer. Predict which forms an anion, which forms a cation, and the charges of each ion. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose. Aluminum Magnesium Hydroxide Chemical Formula.
From www.dreamstime.com
Magnesium Hydroxide Molecular Structure, 3d Model Molecule, Food Additive E528, Structural Aluminum Magnesium Hydroxide Chemical Formula When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution). Aluminum Magnesium Hydroxide Chemical Formula.
From www.alamy.com
3D image of Magnesium hydroxide skeletal formula molecular chemical structure of Aluminum Magnesium Hydroxide Chemical Formula When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. Predict which forms an anion, which forms a cation, and the charges of. Aluminum Magnesium Hydroxide Chemical Formula.
From pharmog.com
Aluminum hydroxide + Magnesium hydroxide + Simethicone chemical Pharmog Aluminum Magnesium Hydroxide Chemical Formula Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. Magnesium and nitrogen react to form an ionic compound. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The lighter group 3a. Aluminum Magnesium Hydroxide Chemical Formula.
From brainly.in
the molecular formula of magnisium hydroxide by criss cross method Brainly.in Aluminum Magnesium Hydroxide Chemical Formula Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer. Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three moles of magnesium [mg] and two moles of. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3. Aluminum Magnesium Hydroxide Chemical Formula.
From www.youtube.com
Aluminium hydroxide magnesium carbonate co. dried gel Labh Group YouTube Aluminum Magnesium Hydroxide Chemical Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Mg + al(oh)3 = mg(oh)2 + al is a single displacement (substitution) reaction where three. Aluminum Magnesium Hydroxide Chemical Formula.
From docslib.org
Aluminum Hydroxide, Magnesium Hydroxide, and Simethicone Memorial Sloan Kettering Cancer Aluminum Magnesium Hydroxide Chemical Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which. Aluminum Magnesium Hydroxide Chemical Formula.
From www.watsons.com.ph
DUCID, Aluminum hydroxide + Magnesium hydroxide 200mg/400mg per 10ml Suspension ANTACID 1 sachet Aluminum Magnesium Hydroxide Chemical Formula Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer. Predict which forms an anion, which forms a cation, and the charges of each ion. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. When an ionic. Aluminum Magnesium Hydroxide Chemical Formula.
From www.youtube.com
How to write Molecular formula of Aluminium hydroxide Chemical formula of Aluminium hydroxide Aluminum Magnesium Hydroxide Chemical Formula Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer. Predict which forms an anion, which forms a cation, and the charges of each ion. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−.. Aluminum Magnesium Hydroxide Chemical Formula.
From chemicalforfo.blogspot.com
Chemical Formula Name Of Magnesium Hydroxide Chemical Formula Info Aluminum Magnesium Hydroxide Chemical Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Magnesium and nitrogen react to form an ionic compound. Aluminium hydroxide, al (oh)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer. Mg + al(oh)3 = mg(oh)2 + al is a. Aluminum Magnesium Hydroxide Chemical Formula.