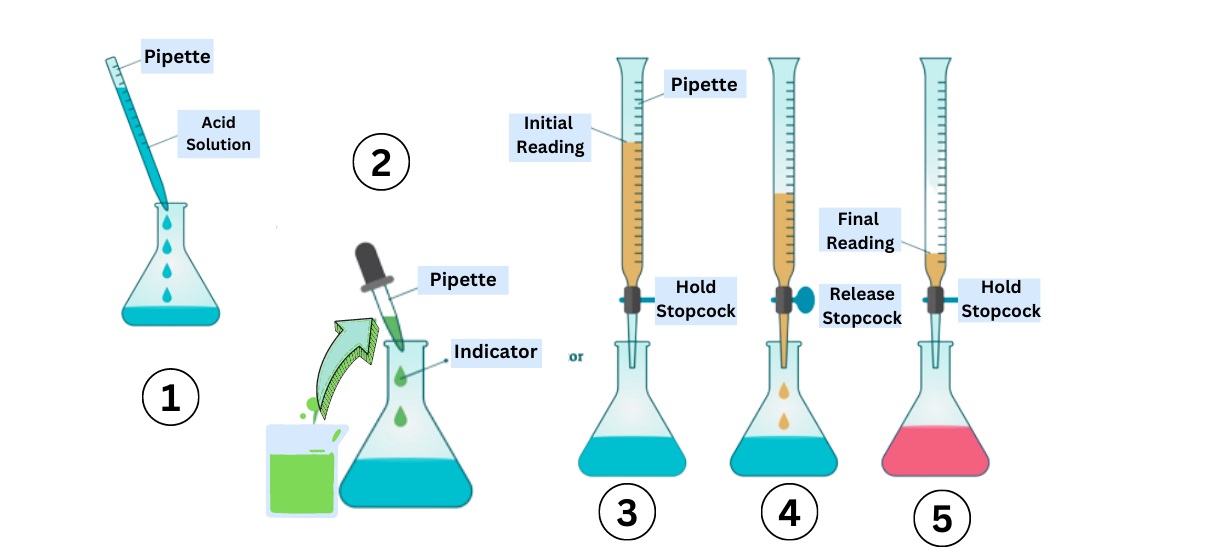
Titration Indicators Lab . You can use a complexometric titration to. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. The reagent (titrant) is the solution with a known molarity that will react with the analyte. This page assumes that you know about ph curves for all the. The analyte (titrand) is the solution with an unknown molarity. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Which indicator is used depends on the chemistry of the reaction taking place between the titrand and. There are many different types of indicators used in titration experiments. You can use a redox titration to identify the amount of vitamin c in your drink. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown.
from ar.inspiredpencil.com
The analyte (titrand) is the solution with an unknown molarity. This page assumes that you know about ph curves for all the. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. You can use a complexometric titration to. You can use a redox titration to identify the amount of vitamin c in your drink. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Which indicator is used depends on the chemistry of the reaction taking place between the titrand and. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. There are many different types of indicators used in titration experiments.
Titration Diagram
Titration Indicators Lab You can use a redox titration to identify the amount of vitamin c in your drink. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. There are many different types of indicators used in titration experiments. You can use a complexometric titration to. This page assumes that you know about ph curves for all the. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. You can use a redox titration to identify the amount of vitamin c in your drink. Which indicator is used depends on the chemistry of the reaction taking place between the titrand and.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Indicators Lab You can use a redox titration to identify the amount of vitamin c in your drink. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. This page assumes that you know about. Titration Indicators Lab.
From slideplayer.com
Chem 106 Class/ Lab Week 12 Sign in / Pick up Papers ppt download Titration Indicators Lab There are many different types of indicators used in titration experiments. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Determine the concentration of analyte present, as well as the acid ionization. Titration Indicators Lab.
From www.studocu.com
06 Indicators Introduction Fall23 LAB Titration Indicators Titration Indicators Lab This page assumes that you know about ph curves for all the. There are many different types of indicators used in titration experiments. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh.. Titration Indicators Lab.
From ar.inspiredpencil.com
Titration Diagram Titration Indicators Lab There are many different types of indicators used in titration experiments. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. The analyte (titrand) is the solution with an unknown molarity. Which indicator. Titration Indicators Lab.
From www.slideshare.net
Lab 3 acid base titration curves and acid_base indicators Titration Indicators Lab Which indicator is used depends on the chemistry of the reaction taking place between the titrand and. The reagent (titrant) is the solution with a known molarity that will react with the analyte. You can use a redox titration to identify the amount of vitamin c in your drink. This figure shows plots of ph versus volume of base added. Titration Indicators Lab.
From www.pinterest.com
Most of us, chemists or otherwise, have probably come across pH Titration Indicators Lab You can use a complexometric titration to. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Which indicator is used depends on the chemistry of the reaction taking place between the titrand and. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color. Titration Indicators Lab.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Indicators Lab You can use a redox titration to identify the amount of vitamin c in your drink. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. There are many different types of indicators. Titration Indicators Lab.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Indicators Lab There are many different types of indicators used in titration experiments. You can use a complexometric titration to. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. The reagent (titrant) is the solution with a known molarity that will react with the analyte. This figure shows plots of ph versus volume of base. Titration Indicators Lab.
From avery-yersbloglester.blogspot.com
Acid Base Titration Lab Report Answers Titration Indicators Lab The reagent (titrant) is the solution with a known molarity that will react with the analyte. This page assumes that you know about ph curves for all the. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. You can use a complexometric titration to. The analyte (titrand) is the solution with an unknown. Titration Indicators Lab.
From www.studocu.com
Titration Indicators escience labs PRELAB QUESTIONS 1. What is the Titration Indicators Lab You can use a redox titration to identify the amount of vitamin c in your drink. You can use a complexometric titration to. Which indicator is used depends on the chemistry of the reaction taking place between the titrand and. This page assumes that you know about ph curves for all the. This figure shows plots of ph versus volume. Titration Indicators Lab.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Titration Indicators Lab Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. The analyte (titrand) is the solution with an unknown molarity. There are many different types of indicators used in titration experiments. This page assumes that you know about ph curves for all the. Which indicator is used depends on the chemistry of the reaction. Titration Indicators Lab.
From www.chegg.com
Solved BUFFERS, TITRATION CURVES, AND INDICATORS LAB REPORT Titration Indicators Lab You can use a complexometric titration to. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. There are many different types of indicators used in titration experiments. The reagent (titrant) is the. Titration Indicators Lab.
From www.reagent.co.uk
Who Invented Titration? The Science Blog Titration Indicators Lab Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. The analyte (titrand) is the solution with an unknown molarity. You can use a redox titration to identify the amount of vitamin c in your drink. There are many different types of indicators used in titration experiments. You can use a complexometric titration to.. Titration Indicators Lab.
From ebinu.blog
AcidBase Titration Lab — DataClassroom / Acid Base Titration Titration Indicators Lab Which indicator is used depends on the chemistry of the reaction taking place between the titrand and. You can use a complexometric titration to. The reagent (titrant) is the solution with a known molarity that will react with the analyte. You can use a redox titration to identify the amount of vitamin c in your drink. Determine the concentration of. Titration Indicators Lab.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Indicators Lab This page assumes that you know about ph curves for all the. Which indicator is used depends on the chemistry of the reaction taking place between the titrand and. There are many different types of indicators used in titration experiments. The reagent (titrant) is the solution with a known molarity that will react with the analyte. You can use a. Titration Indicators Lab.
From chem4three.blogspot.com
CHEMISTRY 11 Titration of Vinegar LAB Titration Indicators Lab You can use a redox titration to identify the amount of vitamin c in your drink. There are many different types of indicators used in titration experiments. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. The analyte (titrand) is the solution with an unknown molarity.. Titration Indicators Lab.
From www.scribd.com
Indicators Titration Chemistry Titration Indicators Lab You can use a redox titration to identify the amount of vitamin c in your drink. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. There are many different types. Titration Indicators Lab.
From mungfali.com
Acid Base Titration Procedure Titration Indicators Lab The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. You can use a redox titration to identify the amount of vitamin c in your drink. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m. Titration Indicators Lab.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Indicators Lab There are many different types of indicators used in titration experiments. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. You can use a redox titration to identify the amount of vitamin. Titration Indicators Lab.
From www.coursehero.com
[Solved] Lab report on Acid base titration Course Hero Titration Indicators Lab You can use a redox titration to identify the amount of vitamin c in your drink. There are many different types of indicators used in titration experiments. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid). Titration Indicators Lab.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Titration Indicators Lab The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. There are many different types of indicators used in titration experiments. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of. Titration Indicators Lab.
From www.chegg.com
Solved EXP. 5 BUFFERS, TITRATION CURVES, AND INDICATORS LAB Titration Indicators Lab There are many different types of indicators used in titration experiments. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown.. Titration Indicators Lab.
From www.studocu.com
Titration Indicators PreLab Questions What is the difference between Titration Indicators Lab Which indicator is used depends on the chemistry of the reaction taking place between the titrand and. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. You can use a redox titration to identify the amount of vitamin c in your drink. The analyte (titrand) is the solution with an unknown molarity. The. Titration Indicators Lab.
From freesvg.org
Redox Titration Using Indicator Free SVG Titration Indicators Lab This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. This page assumes that you know about ph curves for all the. Which indicator is used depends on the chemistry of the reaction. Titration Indicators Lab.
From www.youtube.com
Lab Demonstration Acid Base Titration. YouTube Titration Indicators Lab This page assumes that you know about ph curves for all the. There are many different types of indicators used in titration experiments. Which indicator is used depends on the chemistry of the reaction taking place between the titrand and. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100. Titration Indicators Lab.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Indicators Lab You can use a redox titration to identify the amount of vitamin c in your drink. This page assumes that you know about ph curves for all the. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. You can use a complexometric titration. Titration Indicators Lab.
From mungfali.com
Acid Base Titration Indicator Titration Indicators Lab The analyte (titrand) is the solution with an unknown molarity. You can use a redox titration to identify the amount of vitamin c in your drink. You can use a complexometric titration to. Which indicator is used depends on the chemistry of the reaction taking place between the titrand and. This figure shows plots of ph versus volume of base. Titration Indicators Lab.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Indicators Lab Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. This page assumes that you know about ph curves for all the. Which indicator is used depends on the chemistry of the reaction taking place between the titrand and. You can use a redox titration to identify the amount of vitamin c in your. Titration Indicators Lab.
From www.chegg.com
Solved Titration Indicators 8 5. Identify two reasons why Titration Indicators Lab The reagent (titrant) is the solution with a known molarity that will react with the analyte. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. The analyte (titrand) is the solution with an unknown molarity. Which indicator is used depends on the chemistry of the reaction. Titration Indicators Lab.
From pt.slideshare.net
Lab 3 acid base titration curves and acid_base indicators Titration Indicators Lab There are many different types of indicators used in titration experiments. You can use a complexometric titration to. This page assumes that you know about ph curves for all the. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. Which indicator is used depends on the. Titration Indicators Lab.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Indicators Lab The reagent (titrant) is the solution with a known molarity that will react with the analyte. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. The analyte (titrand) is the solution with. Titration Indicators Lab.
From 38.180.108.73
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Indicators Lab You can use a redox titration to identify the amount of vitamin c in your drink. The analyte (titrand) is the solution with an unknown molarity. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. The reagent (titrant) is the solution with a known molarity that. Titration Indicators Lab.
From www.chegg.com
Solved Titration Indicators EXPERIMENT 1 GETTING AQUAINTED Titration Indicators Lab This page assumes that you know about ph curves for all the. You can use a redox titration to identify the amount of vitamin c in your drink. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. The analyte (titrand) is the solution with an unknown. Titration Indicators Lab.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration Indicators Lab The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. You can use a redox titration to identify the amount of vitamin c in your drink. You can use a complexometric titration to. This figure shows plots of ph versus volume of base added for the titration. Titration Indicators Lab.
From betterlesson.com
Eleventh grade Lesson Titration Lab BetterLesson Titration Indicators Lab This page assumes that you know about ph curves for all the. There are many different types of indicators used in titration experiments. You can use a complexometric titration to. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak. Titration Indicators Lab.