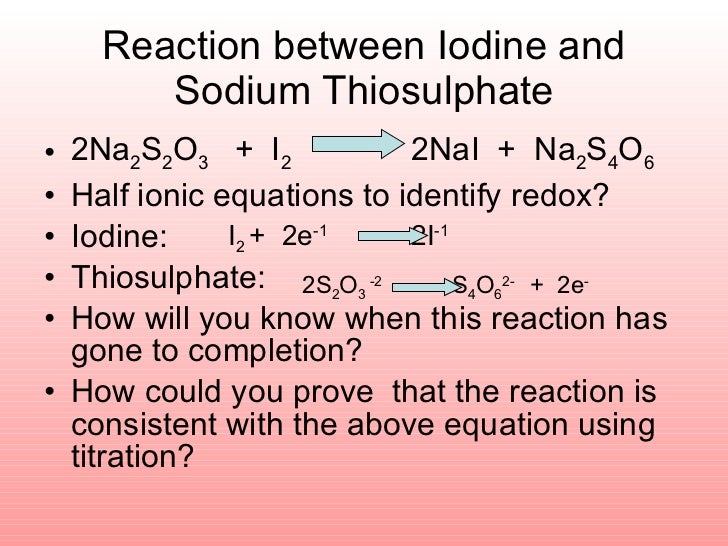
Titration Of Iodine And Sodium Thiosulphate . The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. Iodine, the reaction product, is ordinary titrated with a standard sodium thiosulfate solution, with starch serving as the indicator: Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. A redox reaction occurs between iodine and thiosulfate ions: Iodometry is one of the most important redox titration methods. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. Titration with iodine or thiosulfate. Iodine reacts directly, fast and.
from www.slideshare.net
The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. Titration with iodine or thiosulfate. Iodometry is one of the most important redox titration methods. The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. Iodine, the reaction product, is ordinary titrated with a standard sodium thiosulfate solution, with starch serving as the indicator: A redox reaction occurs between iodine and thiosulfate ions: Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. Iodine reacts directly, fast and. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade.
Writing More Complex Redox Equations
Titration Of Iodine And Sodium Thiosulphate Titration with iodine or thiosulfate. Iodine reacts directly, fast and. Iodometry is one of the most important redox titration methods. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. A redox reaction occurs between iodine and thiosulfate ions: The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. Iodine, the reaction product, is ordinary titrated with a standard sodium thiosulfate solution, with starch serving as the indicator: Titration with iodine or thiosulfate.
From www.alamy.com
Redox titration between iodine and thiosulfate ions. The concentration Titration Of Iodine And Sodium Thiosulphate A redox reaction occurs between iodine and thiosulfate ions: Iodometry is one of the most important redox titration methods. The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. Redox titrations using sodium thiosulphate as a reducing. Titration Of Iodine And Sodium Thiosulphate.
From chempedia.info
Iodine with sodium thiosulphate Big Chemical Encyclopedia Titration Of Iodine And Sodium Thiosulphate The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. Iodometry is one of the most important redox titration methods. Titration with iodine or thiosulfate. A redox reaction occurs between iodine and thiosulfate ions: Redox. Titration Of Iodine And Sodium Thiosulphate.
From www.youtube.com
Iodine and sodium thiosulfate redox titration calculations ALevel Titration Of Iodine And Sodium Thiosulphate Iodometry is one of the most important redox titration methods. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. Iodine reacts directly, fast and. A redox. Titration Of Iodine And Sodium Thiosulphate.
From pixels.com
Iodine And Thiosulfate Ions Titration Photograph by Martyn F. Chillmaid Titration Of Iodine And Sodium Thiosulphate Iodometry is one of the most important redox titration methods. Iodine reacts directly, fast and. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. A redox reaction. Titration Of Iodine And Sodium Thiosulphate.
From www.youtube.com
OCR A level Chemisty Unit F325 Module 3 Redox Titrations Using Titration Of Iodine And Sodium Thiosulphate Iodometry is one of the most important redox titration methods. Titration with iodine or thiosulfate. The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. A redox reaction occurs between iodine and thiosulfate ions: Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep. Titration Of Iodine And Sodium Thiosulphate.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Titration Of Iodine And Sodium Thiosulphate The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. Iodine, the reaction product, is ordinary titrated with a standard sodium thiosulfate solution, with starch serving as the indicator: Iodometry is one of the most important redox titration methods. Iodine reacts directly, fast and. Finally, we add a starch indicator to. Titration Of Iodine And Sodium Thiosulphate.
From www.showme.com
Iodine_ Thiosulfate Titration Science ShowMe Titration Of Iodine And Sodium Thiosulphate Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. Titration with iodine or thiosulfate. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. The freed iodide ions are then titrated using a standardized solution of. Titration Of Iodine And Sodium Thiosulphate.
From www.youtube.com
Iodimetric titration standardization of thiosulfate YouTube Titration Of Iodine And Sodium Thiosulphate Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter. Titration Of Iodine And Sodium Thiosulphate.
From present5.com
Dissolved Oxygen and Biochemical Oxygen Demand Analyses Prepared Titration Of Iodine And Sodium Thiosulphate Iodine reacts directly, fast and. Iodometry is one of the most important redox titration methods. The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. Redox titrations using sodium thiosulphate as a reducing agent is known as. Titration Of Iodine And Sodium Thiosulphate.
From loegibgbt.blob.core.windows.net
Sodium Thiosulfate Iodine Titration at Allene Carter blog Titration Of Iodine And Sodium Thiosulphate The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. Iodine, the reaction product, is ordinary titrated with a standard sodium thiosulfate solution, with starch serving as the indicator:. Titration Of Iodine And Sodium Thiosulphate.
From mavink.com
Sodium Thiosulfate Titration Titration Of Iodine And Sodium Thiosulphate Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. Iodine reacts directly, fast and. A redox reaction occurs between iodine and thiosulfate ions: Iodometry is one of the most important redox titration methods. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since. Titration Of Iodine And Sodium Thiosulphate.
From fphoto.photoshelter.com
science chemistry titration iodine thiosulfate Fundamental Titration Of Iodine And Sodium Thiosulphate As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. Iodine reacts directly, fast and. Iodometry is one of the most important redox titration methods. Finally, we add a starch. Titration Of Iodine And Sodium Thiosulphate.
From fphoto.photoshelter.com
science chemistry titration iodine thiosulfate Fundamental Titration Of Iodine And Sodium Thiosulphate Titration with iodine or thiosulfate. The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. As. Titration Of Iodine And Sodium Thiosulphate.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free Titration Of Iodine And Sodium Thiosulphate The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. Iodine reacts directly, fast and. Iodometry is one of the most important redox titration methods. The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. Titration with iodine or thiosulfate. Redox titrations using sodium thiosulphate as a. Titration Of Iodine And Sodium Thiosulphate.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free Titration Of Iodine And Sodium Thiosulphate As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. A redox reaction occurs between iodine and thiosulfate ions: Iodine reacts directly, fast and. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. The sodium thiosulfate solution. Titration Of Iodine And Sodium Thiosulphate.
From www.youtube.com
Iodine / Thiosulfate Redox Titration Demonstration YouTube Titration Of Iodine And Sodium Thiosulphate Iodine, the reaction product, is ordinary titrated with a standard sodium thiosulfate solution, with starch serving as the indicator: The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine.. Titration Of Iodine And Sodium Thiosulphate.
From studylib.net
An iodine / thiosulfate titration Titration Of Iodine And Sodium Thiosulphate Iodine, the reaction product, is ordinary titrated with a standard sodium thiosulfate solution, with starch serving as the indicator: Titration with iodine or thiosulfate. The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. A redox reaction. Titration Of Iodine And Sodium Thiosulphate.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free Titration Of Iodine And Sodium Thiosulphate Iodine reacts directly, fast and. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. Titration with iodine or thiosulfate. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. Iodine, the reaction product, is ordinary titrated. Titration Of Iodine And Sodium Thiosulphate.
From www.youtube.com
Sodium Thiosulphate Solution preparation and Standardization Titration Of Iodine And Sodium Thiosulphate As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. Iodine reacts directly, fast and. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a. Titration Of Iodine And Sodium Thiosulphate.
From www.youtube.com
Iodine and sodium thiosulfate titrations YouTube Titration Of Iodine And Sodium Thiosulphate A redox reaction occurs between iodine and thiosulfate ions: As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. Iodine reacts directly, fast and. The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. The sodium thiosulfate solution is placed in the burette and, as. Titration Of Iodine And Sodium Thiosulphate.
From www.slideserve.com
PPT Booklet to help with Unit 27 PowerPoint Presentation, free Titration Of Iodine And Sodium Thiosulphate Iodometry is one of the most important redox titration methods. The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is. Titration Of Iodine And Sodium Thiosulphate.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free Titration Of Iodine And Sodium Thiosulphate As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. A redox reaction occurs between iodine and thiosulfate ions: Iodine, the reaction product, is ordinary titrated with a standard sodium thiosulfate solution, with starch serving as the indicator: Iodine reacts directly, fast and. The sodium thiosulfate solution is placed. Titration Of Iodine And Sodium Thiosulphate.
From www.slideshare.net
Writing More Complex Redox Equations Titration Of Iodine And Sodium Thiosulphate Titration with iodine or thiosulfate. The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. Iodine, the reaction product, is ordinary titrated with a standard sodium thiosulfate solution, with starch. Titration Of Iodine And Sodium Thiosulphate.
From exyodfhrb.blob.core.windows.net
Titration Of Iodine Against Sodium Thiosulphate at Jeremiah Ryan blog Titration Of Iodine And Sodium Thiosulphate The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. Iodometry is one of the most important redox titration methods. Iodine reacts directly, fast and. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. Titration with iodine or thiosulfate. A redox reaction occurs between. Titration Of Iodine And Sodium Thiosulphate.
From www.youtube.com
Eureka 2017 Iodometry Redox Titration KIO3 Vs Thiosulfate YouTube Titration Of Iodine And Sodium Thiosulphate Iodine reacts directly, fast and. The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. Iodine, the reaction product, is ordinary titrated with a standard sodium thiosulfate solution, with starch serving as the indicator: Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. A. Titration Of Iodine And Sodium Thiosulphate.
From www.youtube.com
Redox TitrationIodine solution & Sodium Thiosulphate (Na2S2O3) WAEC Titration Of Iodine And Sodium Thiosulphate Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. Titration with iodine or thiosulfate. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. The sodium thiosulfate solution is placed in the burette and, as it. Titration Of Iodine And Sodium Thiosulphate.
From www.youtube.com
N/10 Sodium Thiosulfate Solution Preparation and Standardization with Titration Of Iodine And Sodium Thiosulphate Iodine reacts directly, fast and. The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. A redox reaction occurs between iodine and thiosulfate ions: Iodometry is one of the most important redox titration methods. The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. Redox titrations using. Titration Of Iodine And Sodium Thiosulphate.
From www.slideshare.net
Writing More Complex Redox Equations Titration Of Iodine And Sodium Thiosulphate As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. Iodometry is one of the most important redox titration methods. Iodine, the reaction product, is ordinary titrated with a standard sodium thiosulfate solution, with starch serving as the indicator: A redox reaction occurs between iodine and thiosulfate ions: Titration. Titration Of Iodine And Sodium Thiosulphate.
From byjus.com
Oxidation state of sulphur in sodium thiosulphate during the reaction Titration Of Iodine And Sodium Thiosulphate A redox reaction occurs between iodine and thiosulfate ions: Titration with iodine or thiosulfate. Iodometry is one of the most important redox titration methods. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. Finally, we add a starch indicator to the solution, and the color shifts dramatically from. Titration Of Iodine And Sodium Thiosulphate.
From slideplayer.com
Volumetric Analysis OxidationReduction ppt download Titration Of Iodine And Sodium Thiosulphate Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. Titration with iodine or thiosulfate. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. Iodometry is one of the most important redox titration methods. The sodium. Titration Of Iodine And Sodium Thiosulphate.
From www.toppr.com
.48 Sodium thiosulphate (Na2S2O3 5H20) reacts with iodine as follows Titration Of Iodine And Sodium Thiosulphate A redox reaction occurs between iodine and thiosulfate ions: Iodometry is one of the most important redox titration methods. Iodine reacts directly, fast and. Titration with iodine or thiosulfate. The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. The sodium thiosulfate solution is placed in the burette and, as it is added to the conical. Titration Of Iodine And Sodium Thiosulphate.
From www.youtube.com
ALevel Chemistry Sodium Thiosulfate and Iodine Titrations YouTube Titration Of Iodine And Sodium Thiosulphate As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. Iodine reacts directly, fast and. The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since. Titration Of Iodine And Sodium Thiosulphate.
From www.youtube.com
ALevel Chemistry Sodium Thiosulfate and Iodine titration part 2 YouTube Titration Of Iodine And Sodium Thiosulphate Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. Titration with iodine or thiosulfate. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. The sodium thiosulfate solution is placed in the burette and, as it. Titration Of Iodine And Sodium Thiosulphate.
From fphoto.photoshelter.com
science chemistry titration iodine thiosulfate Fundamental Titration Of Iodine And Sodium Thiosulphate Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. The sodium thiosulfate solution is placed in the burette and, as it is added to the conical flask, it. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to. Titration Of Iodine And Sodium Thiosulphate.
From slideplayer.com
Titration Colour Changes ppt download Titration Of Iodine And Sodium Thiosulphate Iodine, the reaction product, is ordinary titrated with a standard sodium thiosulfate solution, with starch serving as the indicator: Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted. Titration Of Iodine And Sodium Thiosulphate.