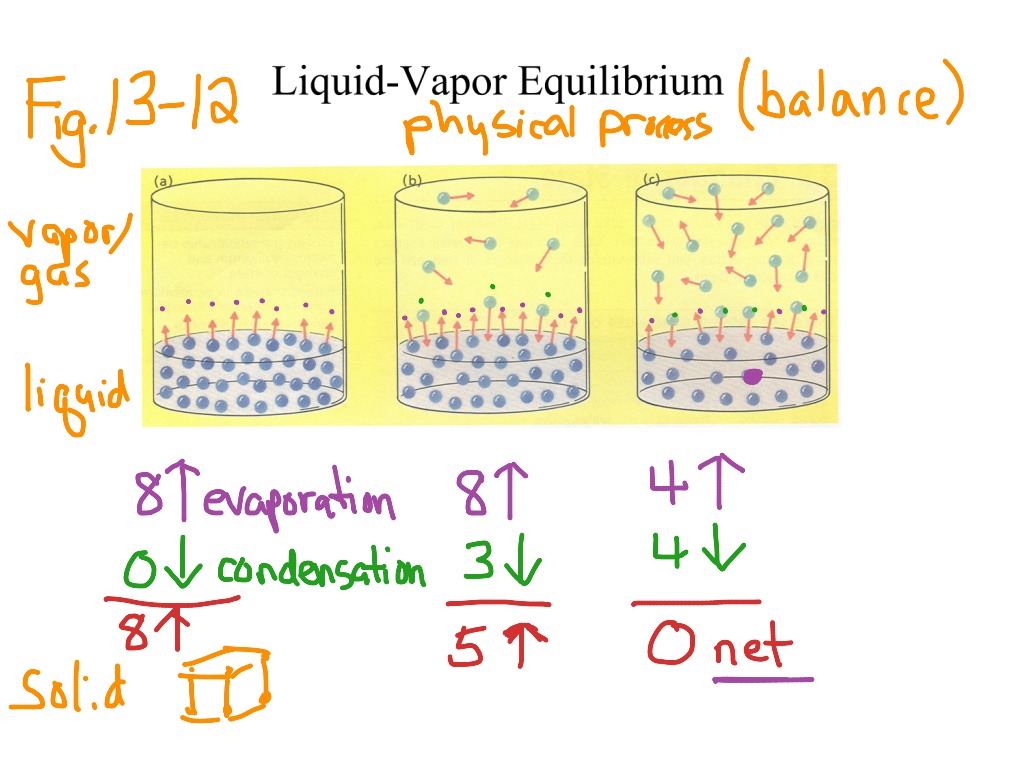
Solid-Liquid Equilibrium In Chemistry . the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for. when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. Mν + xν − (s) ⇌ ν + mz +.
from www.showme.com
consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. Mν + xν − (s) ⇌ ν + mz +. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for.
Liquid Vapor Equilibrium Science, Chemistry, Gases ShowMe
Solid-Liquid Equilibrium In Chemistry the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for. Mν + xν − (s) ⇌ ν + mz +. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions:
From byjus.com
Equilibrium Involving Dissolution Of Solid Gas In Liquid Henry's Law Solid-Liquid Equilibrium In Chemistry the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. Mν + xν − (s) ⇌ ν + mz +. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: By doing so, they generate a population of molecules in the vapor phase above the. Solid-Liquid Equilibrium In Chemistry.
From www.youtube.com
15.5 Heterogeneous Equilibrium Equilibrium Expression Involving a Solid-Liquid Equilibrium In Chemistry simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for. the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. when a liquid is heated, its molecules obtain sufficient. Solid-Liquid Equilibrium In Chemistry.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID Solid-Liquid Equilibrium In Chemistry the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing. Solid-Liquid Equilibrium In Chemistry.
From www.shutterstock.com
Solid Liquid Equilibrium Over 101 RoyaltyFree Licensable Stock Solid-Liquid Equilibrium In Chemistry when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. Mν + xν − (s) ⇌ ν. Solid-Liquid Equilibrium In Chemistry.
From fyonszubj.blob.core.windows.net
Solid Liquid Equilibrium at Thomas McCann blog Solid-Liquid Equilibrium In Chemistry consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for.. Solid-Liquid Equilibrium In Chemistry.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Solid-Liquid Equilibrium In Chemistry By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for. when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape. Solid-Liquid Equilibrium In Chemistry.
From www.showme.com
Liquid Vapor Equilibrium Science, Chemistry, Gases ShowMe Solid-Liquid Equilibrium In Chemistry By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline.. Solid-Liquid Equilibrium In Chemistry.
From studycorgi.com
SolidLiquid Equilibrium in a Binary System Free Essay Example Solid-Liquid Equilibrium In Chemistry By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. Mν + xν − (s) ⇌ ν + mz +. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: the thermodynamics of. Solid-Liquid Equilibrium In Chemistry.
From socratic.org
What do we mean by a dynamic equilibrium? Can you describe how the Solid-Liquid Equilibrium In Chemistry simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase.. Solid-Liquid Equilibrium In Chemistry.
From www.youtube.com
SolidLiquid Chemical Equilibrium YouTube Solid-Liquid Equilibrium In Chemistry the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. Mν + xν − (s) ⇌ ν + mz +. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the. Solid-Liquid Equilibrium In Chemistry.
From ecampusontario.pressbooks.pub
Chemical Equilibrium First Year General Chemistry Solid-Liquid Equilibrium In Chemistry simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. Mν + xν − (s) ⇌ ν + mz +. consider an equilibrium between a crystalline salt (or other kind of ionic solid). Solid-Liquid Equilibrium In Chemistry.
From chem.libretexts.org
11.5 Vapor Pressure Chemistry LibreTexts Solid-Liquid Equilibrium In Chemistry By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. Mν + xν − (s) ⇌ ν + mz +. when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Solid-Liquid Equilibrium In Chemistry.
From mungfali.com
Types Of Equilibrium Chemistry Solid-Liquid Equilibrium In Chemistry when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for.. Solid-Liquid Equilibrium In Chemistry.
From dxoxkxrns.blob.core.windows.net
SolidLiquid Equilibrium Chemistry at Della Wagner blog Solid-Liquid Equilibrium In Chemistry By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for. Mν + xν − (s) ⇌ ν + mz +. the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline.. Solid-Liquid Equilibrium In Chemistry.
From saylordotorg.github.io
Liquids Solid-Liquid Equilibrium In Chemistry the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid. Solid-Liquid Equilibrium In Chemistry.
From socratic.org
In a closed can of soda, dissolved carbonic acid is in equilibrium with Solid-Liquid Equilibrium In Chemistry when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing. Solid-Liquid Equilibrium In Chemistry.
From dxoxkxrns.blob.core.windows.net
SolidLiquid Equilibrium Chemistry at Della Wagner blog Solid-Liquid Equilibrium In Chemistry when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for. consider an equilibrium between a crystalline. Solid-Liquid Equilibrium In Chemistry.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.1.1 The Three States of Matter翰林国际教育 Solid-Liquid Equilibrium In Chemistry By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for. the thermodynamics. Solid-Liquid Equilibrium In Chemistry.
From www.numerade.com
SOLVEDIn a final equilibrium expression solids and liquid have value Solid-Liquid Equilibrium In Chemistry By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. consider an equilibrium between a crystalline. Solid-Liquid Equilibrium In Chemistry.
From studylib.net
Solid Liquid Vapor equilibria phase diagrams Solid-Liquid Equilibrium In Chemistry consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: Mν + xν − (s) ⇌ ν + mz +. the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the. Solid-Liquid Equilibrium In Chemistry.
From learncheme.com
solidsolidliquidphasediagramsconceptestandexampleproblem Solid-Liquid Equilibrium In Chemistry the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for. Mν + xν − (s) ⇌ ν + mz +. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid.. Solid-Liquid Equilibrium In Chemistry.
From ivypanda.com
SolidLiquid Equilibrium in Binary System Test 1922 Words Essay Example Solid-Liquid Equilibrium In Chemistry consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Solid-Liquid Equilibrium In Chemistry.
From fyonszubj.blob.core.windows.net
Solid Liquid Equilibrium at Thomas McCann blog Solid-Liquid Equilibrium In Chemistry the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Solid-Liquid Equilibrium In Chemistry.
From learncheme.com
ssleequilibrium LearnChemE Solid-Liquid Equilibrium In Chemistry when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. Mν + xν − (s) ⇌ ν + mz +. consider an equilibrium between a crystalline salt. Solid-Liquid Equilibrium In Chemistry.
From dxoxkxrns.blob.core.windows.net
SolidLiquid Equilibrium Chemistry at Della Wagner blog Solid-Liquid Equilibrium In Chemistry consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for. when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase.. Solid-Liquid Equilibrium In Chemistry.
From courses.lumenlearning.com
Chemical Equilibria Chemistry for Majors Solid-Liquid Equilibrium In Chemistry when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. the thermodynamics of solid−liquid equilibrium is. Solid-Liquid Equilibrium In Chemistry.
From courses.lumenlearning.com
10.4 Phase Diagrams General College Chemistry I Solid-Liquid Equilibrium In Chemistry when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing. Solid-Liquid Equilibrium In Chemistry.
From kunduz.com
[ANSWERED] Select the incorrect statement For solid liquid equilibrium Solid-Liquid Equilibrium In Chemistry the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for. Mν + xν − (s) ⇌ ν + mz +. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid.. Solid-Liquid Equilibrium In Chemistry.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Solid-Liquid Equilibrium In Chemistry when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor. Solid-Liquid Equilibrium In Chemistry.
From www.toppr.com
Equilibrium in Physical Processes SolidLiquidGas Equilibrium, Examples Solid-Liquid Equilibrium In Chemistry By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. Mν + xν − (s) ⇌ ν. Solid-Liquid Equilibrium In Chemistry.
From www.youtube.com
SolidLiquid Equilibrium YouTube Solid-Liquid Equilibrium In Chemistry the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for. Mν + xν − (s) ⇌ ν + mz +.. Solid-Liquid Equilibrium In Chemistry.
From www.mdpi.com
Molecules Free FullText Modeling of SolidLiquid Equilibria in Solid-Liquid Equilibrium In Chemistry By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome. Solid-Liquid Equilibrium In Chemistry.
From chem.libretexts.org
13.2 Phase Diagrams Binary Systems Chemistry LibreTexts Solid-Liquid Equilibrium In Chemistry the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for. By doing so, they generate a population. Solid-Liquid Equilibrium In Chemistry.
From www.pinterest.com.mx
Download phase_diagram.png image from Chemistry Solid-Liquid Equilibrium In Chemistry simultaneous correlation of liquid−liquid, liquid−solid, and liquid−liquid−solid equilibrium data for. the thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. Mν + xν − (s) ⇌ ν + mz +. when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Solid-Liquid Equilibrium In Chemistry.
From chem.libretexts.org
12.5 SolidLiquid Equilibria Chemistry LibreTexts Solid-Liquid Equilibrium In Chemistry when a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the gaseous phase. By doing so, they generate a population of molecules in the vapor phase above the liquid that produces a pressure—the vapor pressure of the liquid. simultaneous correlation of liquid−liquid, liquid−solid, and. Solid-Liquid Equilibrium In Chemistry.