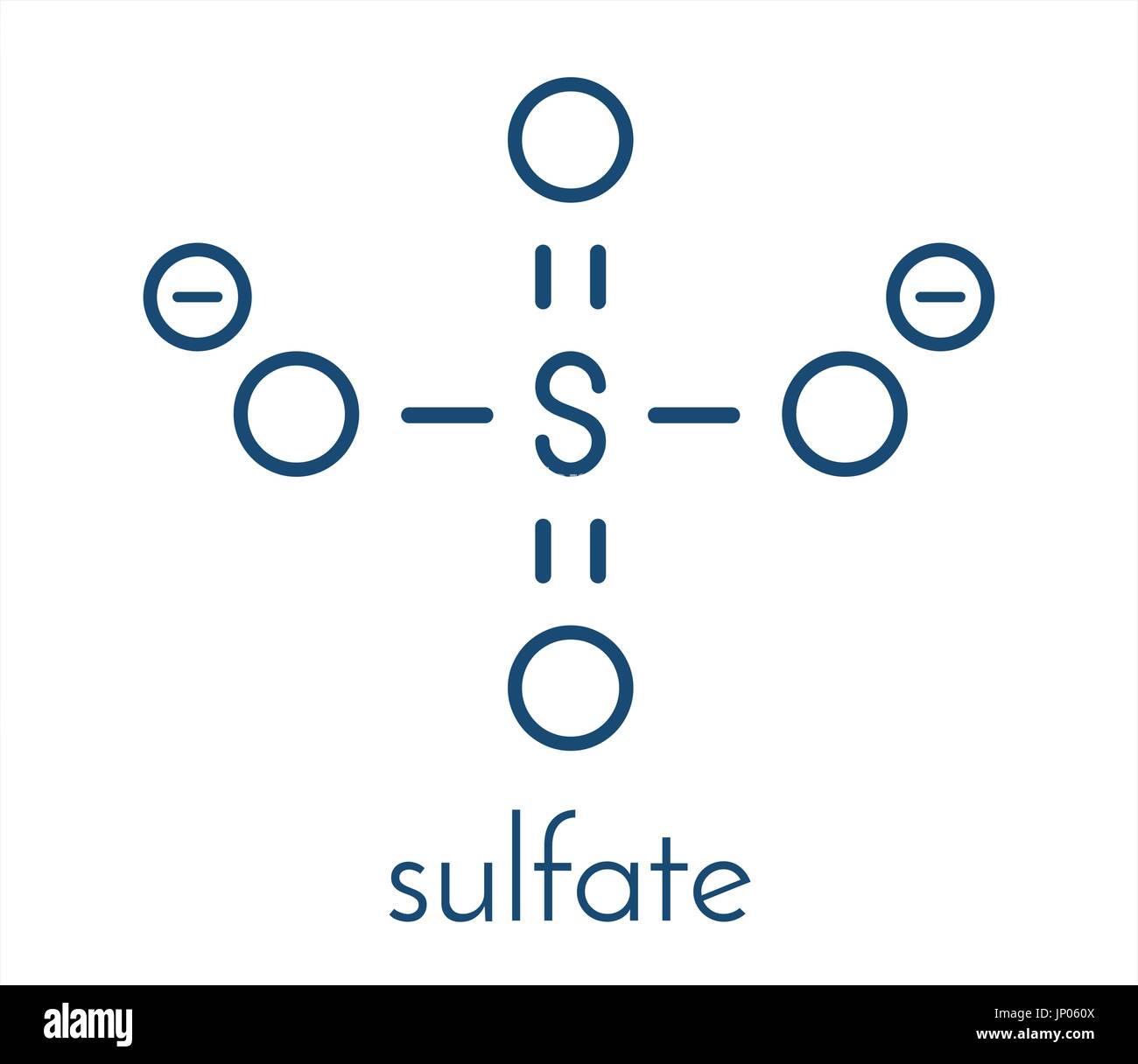
Copper 2 Sulfate Lewis Structure . The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s). Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Find more chemistry widgets in wolfram|alpha. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Binary ionic compounds are between a metal and nonmetal. The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound.
from ar.inspiredpencil.com
Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a. Binary ionic compounds are between a metal and nonmetal. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound. Find more chemistry widgets in wolfram|alpha. The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s).
Copper Lewis Dot Structure
Copper 2 Sulfate Lewis Structure This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound. The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Find more chemistry widgets in wolfram|alpha. The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s). Binary ionic compounds are between a metal and nonmetal. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound.
From www.alamy.com
Copper(II) sulfate with molecular structure. Chemical ingredient used Copper 2 Sulfate Lewis Structure Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. The lewis. Copper 2 Sulfate Lewis Structure.
From ar.inspiredpencil.com
Copper Lewis Dot Structure Copper 2 Sulfate Lewis Structure The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a. Binary ionic compounds are between a metal and nonmetal. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. The lewis structure of copper. Copper 2 Sulfate Lewis Structure.
From www.wikidoc.org
Copper(II) sulfate wikidoc Copper 2 Sulfate Lewis Structure Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s). This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is. Copper 2 Sulfate Lewis Structure.
From www.t3db.ca
T3DB Copper(II) sulfate Copper 2 Sulfate Lewis Structure Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Binary ionic compounds are between a metal and nonmetal. This does not mean there are two atoms, but two types of atoms, so al 2. Copper 2 Sulfate Lewis Structure.
From favpng.com
Copper(II) Sulfate Hydrate Crystal Structure, PNG, 1807x2150px Copper 2 Sulfate Lewis Structure The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s). Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound. The. Copper 2 Sulfate Lewis Structure.
From www.fishersci.co.uk
Copper(II) sulfate hydrate, Puratronic , 99.999 (metals basis), Thermo Copper 2 Sulfate Lewis Structure Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Binary ionic. Copper 2 Sulfate Lewis Structure.
From science.wonderhowto.com
How to Draw the Lewis dot structure for sulfate « Science Experiments Copper 2 Sulfate Lewis Structure This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound. Find more chemistry widgets in wolfram|alpha. The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a. Binary ionic compounds are between. Copper 2 Sulfate Lewis Structure.
From ar.inspiredpencil.com
Copper Ii Sulfate Pentahydrate Anhydrous Copper 2 Sulfate Lewis Structure The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s). The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a. Find more chemistry widgets in wolfram|alpha. This does not mean there are two. Copper 2 Sulfate Lewis Structure.
From molekula.com
Purchase Copper(II) sulfate anhydrous [7758987] online • Catalog Copper 2 Sulfate Lewis Structure The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s). The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a. Binary ionic compounds are between a metal and nonmetal. This does not mean. Copper 2 Sulfate Lewis Structure.
From www.sciencephoto.com
Copper (II) sulphate forms Stock Image C004/7693 Science Photo Copper 2 Sulfate Lewis Structure Binary ionic compounds are between a metal and nonmetal. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound. The lewis structure of copper sulfate, cuso₄, contains. Copper 2 Sulfate Lewis Structure.
From www.alamy.com
3D image of Copper II sulfate skeletal formula molecular chemical Copper 2 Sulfate Lewis Structure Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Binary ionic compounds are between a metal and nonmetal. The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion. Copper 2 Sulfate Lewis Structure.
From favpng.com
Copper(II) Sulfate Crystal Structure, PNG, 1092x914px, Copperii Sulfate Copper 2 Sulfate Lewis Structure This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound. Find more chemistry widgets in wolfram|alpha. Binary ionic compounds are between a metal and nonmetal. The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s). Copper. Copper 2 Sulfate Lewis Structure.
From drivenheisenberg.blogspot.com
Lewis Dot Diagram For Copper Drivenheisenberg Copper 2 Sulfate Lewis Structure Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s). Binary ionic compounds are between a metal and nonmetal. The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper. Copper 2 Sulfate Lewis Structure.
From www.youtube.com
How to Write the Formula for Copper (II) sulfate YouTube Copper 2 Sulfate Lewis Structure This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound. Find more chemistry widgets in wolfram|alpha. The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a. Binary ionic compounds are between. Copper 2 Sulfate Lewis Structure.
From commons.wikimedia.org
FileCopper(II)sulfatepentahydrateCu1coordxtal2007CM3Dballs Copper 2 Sulfate Lewis Structure The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a. The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s). Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical. Copper 2 Sulfate Lewis Structure.
From favpng.com
Copper(II) Sulfate Crystal Structure, PNG, 2112x2150px, Copperii Copper 2 Sulfate Lewis Structure The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a. The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s). Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical. Copper 2 Sulfate Lewis Structure.
From www.alamy.com
Copper(II) sulfate with chemical formula. Chemical ingredient used in Copper 2 Sulfate Lewis Structure The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound. Find more chemistry widgets in wolfram|alpha. Binary ionic compounds are between. Copper 2 Sulfate Lewis Structure.
From www.rcilabscan.com
Copper (II) Sulfate Pentahydrate, AR RCI LABSCAN LIMITED (EN) Copper 2 Sulfate Lewis Structure Binary ionic compounds are between a metal and nonmetal. Find more chemistry widgets in wolfram|alpha. The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s). Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. The structure of copper(ii). Copper 2 Sulfate Lewis Structure.
From www.pngwing.com
Copper(II) sulfate Crystal structure Chemical compound, salt, natural Copper 2 Sulfate Lewis Structure Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound. The lewis. Copper 2 Sulfate Lewis Structure.
From www.pngegg.com
Copper(II) sulfate Crystal structure, crystal ball, angle, text png Copper 2 Sulfate Lewis Structure The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s). Find more chemistry widgets in wolfram|alpha. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Get the free lewis structure finder widget for your website, blog, wordpress, blogger,. Copper 2 Sulfate Lewis Structure.
From www.sciencephoto.com
Hydrated copper (II) sulphate Stock Image C009/9431 Science Photo Copper 2 Sulfate Lewis Structure This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Binary ionic compounds are between a metal and nonmetal. Find more chemistry widgets in wolfram|alpha. The structure. Copper 2 Sulfate Lewis Structure.
From www.wikidoc.org
Copper(II) sulfate wikidoc Copper 2 Sulfate Lewis Structure Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound. The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion,. Copper 2 Sulfate Lewis Structure.
From www.youtube.com
Lewis Structure of CuS, copper (II) sulfide YouTube Copper 2 Sulfate Lewis Structure The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a. Binary ionic compounds are between a metal and nonmetal. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. This does not mean there are two atoms, but two. Copper 2 Sulfate Lewis Structure.
From www.glentham.com
Copper(II) sulfate pentahydrate (CAS 7758998) Glentham Life Sciences Copper 2 Sulfate Lewis Structure Binary ionic compounds are between a metal and nonmetal. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. This does not mean there are two atoms, but two types of atoms, so al 2. Copper 2 Sulfate Lewis Structure.
From www.pinterest.co.uk
Iron And Copper Sulfate Reaction Chemical Sodium, Nats, Biochemistry Copper 2 Sulfate Lewis Structure Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Binary ionic compounds are between a metal and nonmetal. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound. The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a. Copper 2 Sulfate Lewis Structure.
From www.dreamstime.com
Copper(II) Sulfate with Molecular Structure. Chemical Ingredient Used Copper 2 Sulfate Lewis Structure Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Binary ionic compounds are between a metal and nonmetal. Find more chemistry widgets in wolfram|alpha. The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s). Copper (ii) sulfate, also known as cupric sulfate, is. Copper 2 Sulfate Lewis Structure.
From www.alamy.com
Copper(II) sulfate with molecular structure. Chemical ingredient used Copper 2 Sulfate Lewis Structure The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. This does not mean there are two atoms, but two types of atoms, so. Copper 2 Sulfate Lewis Structure.
From www.fishersci.no
Copper(II) sulfate pentahydrate, 98+, ACS reagent, Thermo Scientific Copper 2 Sulfate Lewis Structure Binary ionic compounds are between a metal and nonmetal. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s). Find more chemistry widgets in wolfram|alpha. This does not mean there are two atoms, but. Copper 2 Sulfate Lewis Structure.
From www.fishersci.se
Ammonium copper(II) sulfate hexahydrate, 99, Thermo Scientific Copper 2 Sulfate Lewis Structure Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s). Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. The structure of copper(ii). Copper 2 Sulfate Lewis Structure.
From www.vrogue.co
How To Write The Formula For Copper Ii Sulfate Youtub vrogue.co Copper 2 Sulfate Lewis Structure Find more chemistry widgets in wolfram|alpha. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the. Copper 2 Sulfate Lewis Structure.
From www.guidechem.com
AMMONIUM COPPER(II) SULFATE HEXAHYDRATE 13587263 wiki Copper 2 Sulfate Lewis Structure The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a. The lewis structure of copper sulfate, cuso₄, contains one copper (cu²⁺) ion and one sulfate (so₄²⁻) ion, where the sulfur (s). This does not mean there are two atoms, but two types of atoms,. Copper 2 Sulfate Lewis Structure.
From www.pngegg.com
Copper(II) sulfate Crystal structure, crystal ball, angle, text png Copper 2 Sulfate Lewis Structure The structure of copper(ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. This does not mean there are two atoms, but two types of atoms, so. Copper 2 Sulfate Lewis Structure.
From www.youtube.com
How to Draw the Lewis Dot Structure for CuSO4 Copper (II) sulfate Copper 2 Sulfate Lewis Structure This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Find more chemistry widgets in wolfram|alpha. Binary ionic compounds are between a metal and nonmetal. The structure. Copper 2 Sulfate Lewis Structure.
From www.youtube.com
Color of Copper (II) sulfate [hydrous and anhydrous] YouTube Copper 2 Sulfate Lewis Structure Find more chemistry widgets in wolfram|alpha. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary ionic compound. Binary ionic compounds are between a metal and nonmetal. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Copper (ii) sulfate, also known as cupric. Copper 2 Sulfate Lewis Structure.