Beer's Law Mcat . In other words, a solution absorbs more. Pretty useful and super easy. Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength of greatest absorbance), c is the. What are the applications of beer's law? Absorbance = some constant x length of path traveled by light x solution concentration. In what situations do researchers measure absorbance? In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. I know absorbance is measured to determine. I can see it being used in microbiology to quantitatively measure turbidity for bacterial growth. 10k+ visitors in the past month Really can be applied to a lot. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as the beer.
from www.numerade.com
In what situations do researchers measure absorbance? I know absorbance is measured to determine. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as the beer. Pretty useful and super easy. Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength of greatest absorbance), c is the. I can see it being used in microbiology to quantitatively measure turbidity for bacterial growth. What are the applications of beer's law? In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Really can be applied to a lot. 10k+ visitors in the past month
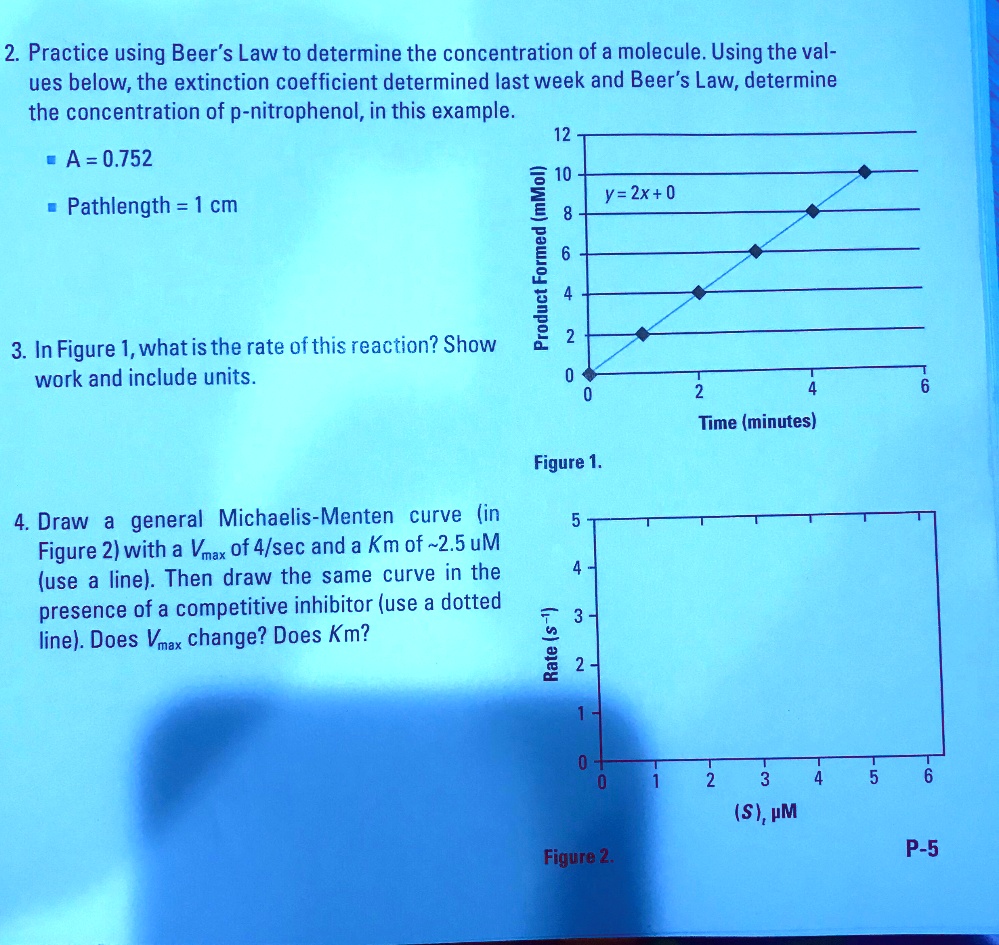
SOLVED 2 Practice using Beer's Law to determine the concentration of a molecule. Using the val
Beer's Law Mcat In other words, a solution absorbs more. Really can be applied to a lot. I can see it being used in microbiology to quantitatively measure turbidity for bacterial growth. What are the applications of beer's law? Absorbance = some constant x length of path traveled by light x solution concentration. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. I know absorbance is measured to determine. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as the beer. 10k+ visitors in the past month Pretty useful and super easy. In other words, a solution absorbs more. Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength of greatest absorbance), c is the. In what situations do researchers measure absorbance?
From app.formative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Beer's Law Mcat In what situations do researchers measure absorbance? I know absorbance is measured to determine. Absorbance = some constant x length of path traveled by light x solution concentration. I can see it being used in microbiology to quantitatively measure turbidity for bacterial growth. In other words, a solution absorbs more. 10k+ visitors in the past month In spectroscopy, beer’s law. Beer's Law Mcat.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Mcat Pretty useful and super easy. I know absorbance is measured to determine. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. 10k+ visitors in the past month What are the applications of beer's law? Beer's law is expressed in equation 1.3, where e is. Beer's Law Mcat.
From www.researchgate.net
Beer's law verification range for method A (a) and Method B (b) Download Scientific Diagram Beer's Law Mcat Really can be applied to a lot. I can see it being used in microbiology to quantitatively measure turbidity for bacterial growth. Absorbance = some constant x length of path traveled by light x solution concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known. Beer's Law Mcat.
From www.researchgate.net
Beer's law curves for method A and method B Download Scientific Diagram Beer's Law Mcat In what situations do researchers measure absorbance? I know absorbance is measured to determine. Pretty useful and super easy. Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength of greatest absorbance), c is the. 10k+ visitors in the past month In spectroscopy, beer’s law states that the absorption of light. Beer's Law Mcat.
From openlab.citytech.cuny.edu
Beer’s Law Biology 1101 Course Hub Beer's Law Mcat I can see it being used in microbiology to quantitatively measure turbidity for bacterial growth. 10k+ visitors in the past month Absorbance = some constant x length of path traveled by light x solution concentration. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration.. Beer's Law Mcat.
From www.coursehero.com
[Solved] When you turn beers law into y=mx+b, what components of beers law... Course Hero Beer's Law Mcat Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as the beer. Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength of greatest absorbance), c is the. In spectroscopy, beer’s law states that the absorption. Beer's Law Mcat.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer's Law Mcat 10k+ visitors in the past month I can see it being used in microbiology to quantitatively measure turbidity for bacterial growth. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution absorbs more. What are the applications of beer's law?. Beer's Law Mcat.
From www.coursehero.com
Beer's Law.docx Beer’s Law By Amanda Katen Chemistry 116 May 10, 2020 Exercise 1 Colorimeter Beer's Law Mcat Really can be applied to a lot. What are the applications of beer's law? Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as the beer. In what situations do researchers measure absorbance? In spectroscopy, beer’s law states that the absorption of light by a. Beer's Law Mcat.
From www.chegg.com
Solved Simulation Data and Report Submission Beer's Law Beer's Law Mcat Really can be applied to a lot. Absorbance = some constant x length of path traveled by light x solution concentration. 10k+ visitors in the past month What are the applications of beer's law? I know absorbance is measured to determine. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the. Beer's Law Mcat.
From www.youtube.com
Beer's Law Unknown Calculation Find Grams of Unknown (57) YouTube Beer's Law Mcat What are the applications of beer's law? Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as the beer. Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength of greatest absorbance), c is the. In. Beer's Law Mcat.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 from Advanced Chemistry Beer's Law Mcat In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Pretty useful and super easy. Really can be applied to a lot. In what situations do researchers measure absorbance? Absorbance = some constant x length of path traveled by light x solution concentration. 10k+ visitors. Beer's Law Mcat.
From www.youtube.com
MCAT Test Prep Radiochemistry & Spectroscopy. UV, IR, NMR, Beer's Law Beer's Law Mcat In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as the beer. Absorbance = some constant x length of path. Beer's Law Mcat.
From www.slideserve.com
PPT Determination of Concentration Using Spectrophotometry PowerPoint Presentation ID2435454 Beer's Law Mcat In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. I can see it being used in microbiology to quantitatively measure turbidity for bacterial growth. In what situations do researchers measure absorbance? Since the concentration, path length and molar absorptivity are all directly proportional to. Beer's Law Mcat.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Mcat I know absorbance is measured to determine. Absorbance = some constant x length of path traveled by light x solution concentration. Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength of greatest absorbance), c is the. I can see it being used in microbiology to quantitatively measure turbidity for bacterial. Beer's Law Mcat.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Mcat Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as the beer. Really can be applied to a lot. In what situations do researchers measure absorbance? What are the applications of beer's law? I can see it being used in microbiology to quantitatively measure turbidity. Beer's Law Mcat.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer's Law Mcat Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as the beer. In other words, a solution absorbs more. Pretty useful and super easy. Absorbance = some constant x length of path traveled by light x solution concentration. In spectroscopy, beer’s law states that the. Beer's Law Mcat.
From brainly.in
Beer lambert law formula Brainly.in Beer's Law Mcat I can see it being used in microbiology to quantitatively measure turbidity for bacterial growth. I know absorbance is measured to determine. In other words, a solution absorbs more. In what situations do researchers measure absorbance? Absorbance = some constant x length of path traveled by light x solution concentration. Since the concentration, path length and molar absorptivity are all. Beer's Law Mcat.
From www.numerade.com
SOLVED Consider the Beer's Law equation A = εbc. Match the variable with the correct Beer's Law Mcat I can see it being used in microbiology to quantitatively measure turbidity for bacterial growth. Absorbance = some constant x length of path traveled by light x solution concentration. Pretty useful and super easy. 10k+ visitors in the past month In what situations do researchers measure absorbance? Beer's law is expressed in equation 1.3, where e is a constant for. Beer's Law Mcat.
From www.chegg.com
Solved SPECTROPHOTOMETRY APPLY BEER'S LAW TO DETERMINE Beer's Law Mcat Absorbance = some constant x length of path traveled by light x solution concentration. Pretty useful and super easy. I can see it being used in microbiology to quantitatively measure turbidity for bacterial growth. Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength of greatest absorbance), c is the. What. Beer's Law Mcat.
From www.numerade.com
SOLVED 2 Practice using Beer's Law to determine the concentration of a molecule. Using the val Beer's Law Mcat I know absorbance is measured to determine. Absorbance = some constant x length of path traveled by light x solution concentration. I can see it being used in microbiology to quantitatively measure turbidity for bacterial growth. In what situations do researchers measure absorbance? Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. Beer's Law Mcat.
From www.studypool.com
SOLUTION Beer S Law Simulation Studypool Beer's Law Mcat Really can be applied to a lot. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as the beer. Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength of greatest absorbance), c is the. Absorbance. Beer's Law Mcat.
From www.softpedia.com
Download Beer's Law Lab 1.02 Beer's Law Mcat Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength of greatest absorbance), c is the. Pretty useful and super easy. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as the beer. In spectroscopy, beer’s. Beer's Law Mcat.
From www.researchgate.net
Beer's law plot of second derivative data [HMBATSC] = 1 x 103 M... Download Scientific Diagram Beer's Law Mcat In what situations do researchers measure absorbance? In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as the beer. What. Beer's Law Mcat.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Mcat What are the applications of beer's law? Absorbance = some constant x length of path traveled by light x solution concentration. Really can be applied to a lot. I know absorbance is measured to determine. Pretty useful and super easy. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of. Beer's Law Mcat.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Mcat Absorbance = some constant x length of path traveled by light x solution concentration. I know absorbance is measured to determine. 10k+ visitors in the past month In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution absorbs more. I. Beer's Law Mcat.
From www.studocu.com
Beer's Law Experiment Experiment 4 Beer’s Law. Purpose The purpose of this lab is to Beer's Law Mcat Absorbance = some constant x length of path traveled by light x solution concentration. I can see it being used in microbiology to quantitatively measure turbidity for bacterial growth. 10k+ visitors in the past month In what situations do researchers measure absorbance? What are the applications of beer's law? Pretty useful and super easy. In spectroscopy, beer’s law states that. Beer's Law Mcat.
From www.chegg.com
Solved a. ( 3 pts.) Use Beer's Law and the directions above Beer's Law Mcat In other words, a solution absorbs more. Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength of greatest absorbance), c is the. Pretty useful and super easy. I can see it being used in microbiology to quantitatively measure turbidity for bacterial growth. Absorbance = some constant x length of path. Beer's Law Mcat.
From www.softpedia.com
Download Beer's Law Lab Beer's Law Mcat Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as the beer. Absorbance = some constant x length of path traveled by light x solution concentration. 10k+ visitors in the past month In spectroscopy, beer’s law states that the absorption of light by a sample. Beer's Law Mcat.
From www.studocu.com
Chem 181 4 Beer's Law Beer Lab Pre lab Determining the Concentration of a Solution Beer’s Beer's Law Mcat Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength of greatest absorbance), c is the. Absorbance = some constant x length of path traveled by light x solution concentration. Really can be applied to a lot. In what situations do researchers measure absorbance? I know absorbance is measured to determine.. Beer's Law Mcat.
From studylib.net
Beer's Law Lab Beer's Law Mcat In other words, a solution absorbs more. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. What are the applications of beer's law? Absorbance = some constant x length of path traveled by light x solution concentration. Since the concentration, path length and molar. Beer's Law Mcat.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID2230605 Beer's Law Mcat Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength of greatest absorbance), c is the. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. What are the applications of beer's law? I can see. Beer's Law Mcat.
From www.coursehero.com
[Solved] What factors are included in the Beer's law expression for... Course Hero Beer's Law Mcat Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength of greatest absorbance), c is the. Really can be applied to a lot. Pretty useful and super easy. In what situations do researchers measure absorbance? Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we. Beer's Law Mcat.
From www.studocu.com
Beers Law Lab Ph ET Student Sheet AP Chemistry PhET Simulation Beer’s Law Lab Learning Goals Beer's Law Mcat Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength of greatest absorbance), c is the. What are the applications of beer's law? I know absorbance is measured to determine. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its. Beer's Law Mcat.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer's Law Mcat Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as the beer. In what situations do researchers measure absorbance? What are the applications of beer's law? Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength. Beer's Law Mcat.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement... Download Scientific Beer's Law Mcat Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as the beer. Beer's law is expressed in equation 1.3, where e is a constant for the solute at lamdamax (the wavelength of greatest absorbance), c is the. In other words, a solution absorbs more. I. Beer's Law Mcat.