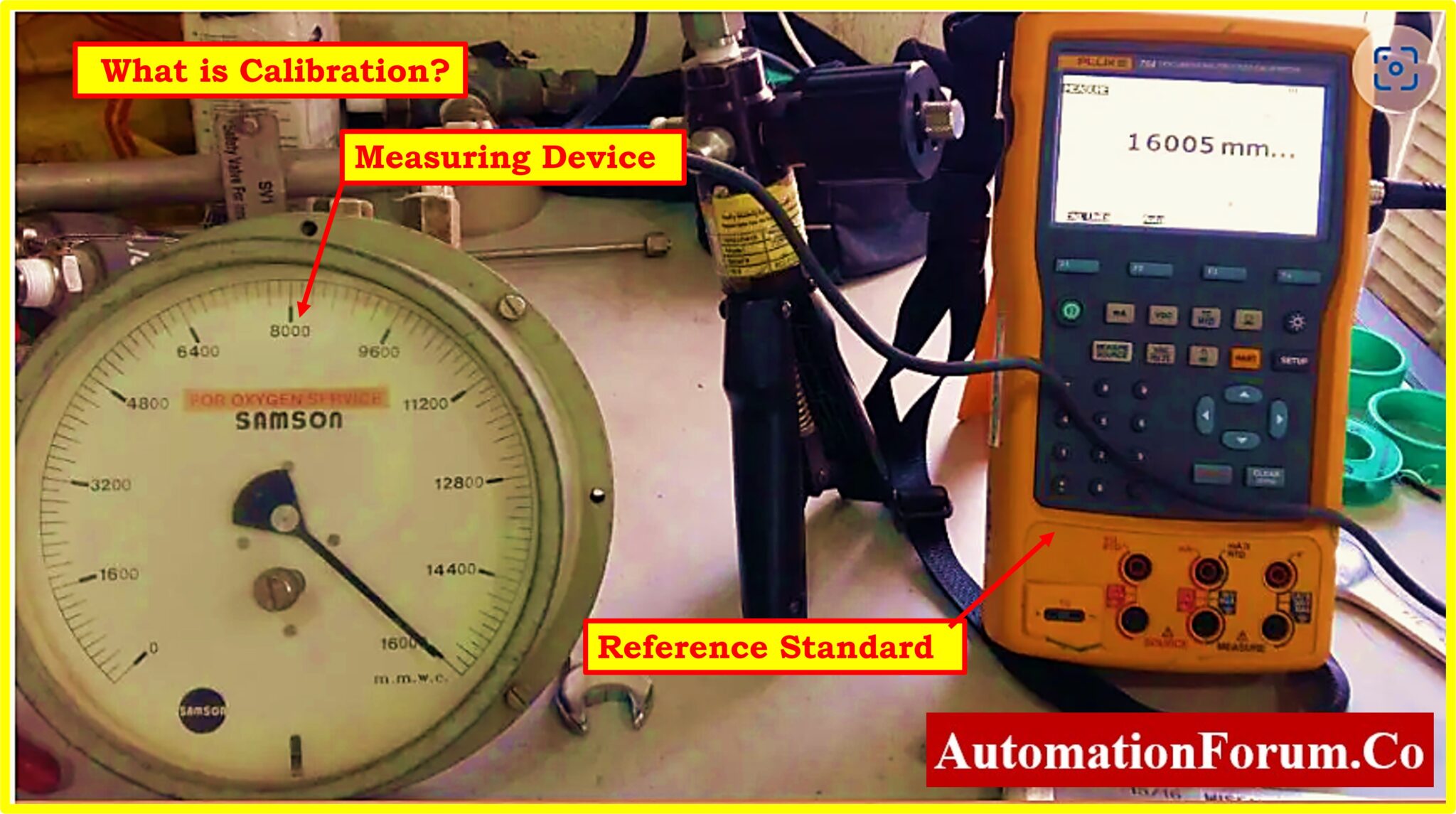
Calibration Definition Fda . calibration requirements for equipment are defined by title 21 of the fda’s code of federal regulations. Concomitant material refer to those. fda amended the definition of manufacturing material to help clarify this definition. § 58.63 maintenance and calibration of equipment. Calibration procedures shall include specific directions and limits for accuracy and precision. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. once drug development reaches the stage where the api is produced for use in drug products intended for. (a) there shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug. (a) equipment shall be adequately inspected, cleaned, and. Calibration procedures shall include specific directions and limits for accuracy and precision. The cgmp regulations for validating pharmaceutical.
from automationforum.co
calibration requirements for equipment are defined by title 21 of the fda’s code of federal regulations. The cgmp regulations for validating pharmaceutical. § 58.63 maintenance and calibration of equipment. (a) equipment shall be adequately inspected, cleaned, and. Calibration procedures shall include specific directions and limits for accuracy and precision. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. Calibration procedures shall include specific directions and limits for accuracy and precision. fda amended the definition of manufacturing material to help clarify this definition. Concomitant material refer to those. (a) there shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug.
Differences Between Validation and Calibration
Calibration Definition Fda Calibration procedures shall include specific directions and limits for accuracy and precision. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. Calibration procedures shall include specific directions and limits for accuracy and precision. fda amended the definition of manufacturing material to help clarify this definition. calibration requirements for equipment are defined by title 21 of the fda’s code of federal regulations. (a) equipment shall be adequately inspected, cleaned, and. once drug development reaches the stage where the api is produced for use in drug products intended for. Calibration procedures shall include specific directions and limits for accuracy and precision. The cgmp regulations for validating pharmaceutical. Concomitant material refer to those. (a) there shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug. § 58.63 maintenance and calibration of equipment.
From blog.beamex.com
The most common calibrationrelated FDA warnings to pharma companies Calibration Definition Fda The cgmp regulations for validating pharmaceutical. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. Calibration procedures shall include specific directions and limits for accuracy and precision. (a) equipment shall be adequately inspected, cleaned, and. § 58.63 maintenance and calibration of equipment. once drug development reaches the stage where the api. Calibration Definition Fda.
From www.youtube.com
Calibration Methods in Analytical Chemistry YouTube Calibration Definition Fda once drug development reaches the stage where the api is produced for use in drug products intended for. The cgmp regulations for validating pharmaceutical. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. Calibration procedures shall include specific directions and limits for accuracy and precision. Concomitant material refer to those. calibration. Calibration Definition Fda.
From www.xenia.team
Calibrate Food Thermometer Poster Free Tools by Xenia Calibration Definition Fda calibration requirements for equipment are defined by title 21 of the fda’s code of federal regulations. (a) equipment shall be adequately inspected, cleaned, and. The cgmp regulations for validating pharmaceutical. fda amended the definition of manufacturing material to help clarify this definition. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers.. Calibration Definition Fda.
From www.medicaldesignbriefs.com
Guide to FDA Requirements and Importance of Medical Device Calibration Calibration Definition Fda once drug development reaches the stage where the api is produced for use in drug products intended for. Concomitant material refer to those. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. Calibration procedures shall include specific directions and limits for accuracy and precision. The cgmp regulations for validating pharmaceutical. calibration. Calibration Definition Fda.
From chem-net.blogspot.com
What is calibration? Calibrated instrumentsAnalytical Chemistry Calibration Definition Fda Calibration procedures shall include specific directions and limits for accuracy and precision. (a) there shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug. (a) equipment shall be adequately inspected, cleaned, and. Concomitant material refer to those. once drug development reaches the stage where the api is produced. Calibration Definition Fda.
From automationforum.co
Differences Between Validation and Calibration Calibration Definition Fda § 58.63 maintenance and calibration of equipment. (a) there shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug. calibration requirements for equipment are defined by title 21 of the fda’s code of federal regulations. (a) equipment shall be adequately inspected, cleaned, and. The cgmp regulations for. Calibration Definition Fda.
From engineeringlearn.com
Types of Calibration Definition, Purpose, Instrument & Examples Calibration Definition Fda fda amended the definition of manufacturing material to help clarify this definition. § 58.63 maintenance and calibration of equipment. Calibration procedures shall include specific directions and limits for accuracy and precision. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. (a) equipment shall be adequately inspected, cleaned, and. The cgmp regulations. Calibration Definition Fda.
From www.youtube.com
What is calibration? Definition, Importance, Types, Affects, Uses Calibration Definition Fda once drug development reaches the stage where the api is produced for use in drug products intended for. Calibration procedures shall include specific directions and limits for accuracy and precision. The cgmp regulations for validating pharmaceutical. Calibration procedures shall include specific directions and limits for accuracy and precision. (a) equipment shall be adequately inspected, cleaned, and. calibration requirements. Calibration Definition Fda.
From pharmaguddu.com
Difference Between Validation, Calibration, and Qualification in Pharma Calibration Definition Fda (a) equipment shall be adequately inspected, cleaned, and. Calibration procedures shall include specific directions and limits for accuracy and precision. The cgmp regulations for validating pharmaceutical. calibration requirements for equipment are defined by title 21 of the fda’s code of federal regulations. (a) there shall be a quality control unit that shall have the responsibility and authority to. Calibration Definition Fda.
From www.youtube.com
Calibration of measuring instruments Definition of calibration Calibration Definition Fda The cgmp regulations for validating pharmaceutical. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. once drug development reaches the stage where the api is produced for use in drug products intended for. (a) equipment shall be adequately inspected, cleaned, and. calibration requirements for equipment are defined by title 21 of. Calibration Definition Fda.
From engineeringlearn.com
Types of Calibration Definition, Purpose, Instrument & Examples Calibration Definition Fda fda amended the definition of manufacturing material to help clarify this definition. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. calibration requirements for equipment are defined by title 21 of the fda’s code of federal regulations. Concomitant material refer to those. (a) there shall be a quality control unit. Calibration Definition Fda.
From engineeringlearn.com
Types of Calibration Definition, Purpose, Instrument & Examples Calibration Definition Fda Concomitant material refer to those. (a) equipment shall be adequately inspected, cleaned, and. Calibration procedures shall include specific directions and limits for accuracy and precision. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. § 58.63 maintenance and calibration of equipment. (a) there shall be a quality control unit that shall. Calibration Definition Fda.
From www.youtube.com
What is Device Calibration and Why is it So Important? YouTube Calibration Definition Fda Calibration procedures shall include specific directions and limits for accuracy and precision. fda amended the definition of manufacturing material to help clarify this definition. (a) there shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug. once drug development reaches the stage where the api is produced. Calibration Definition Fda.
From www.omaada.com
Understanding Instrument Calibration Definition and Significance Calibration Definition Fda Calibration procedures shall include specific directions and limits for accuracy and precision. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. (a) equipment shall be adequately inspected, cleaned, and. The cgmp regulations for validating pharmaceutical. once drug development reaches the stage where the api is produced for use in drug products intended. Calibration Definition Fda.
From www.shoebox.md
Biological Calibration of Audiometers A Comprehensive Guide Calibration Definition Fda once drug development reaches the stage where the api is produced for use in drug products intended for. § 58.63 maintenance and calibration of equipment. Calibration procedures shall include specific directions and limits for accuracy and precision. (a) equipment shall be adequately inspected, cleaned, and. calibration requirements for equipment are defined by title 21 of the fda’s. Calibration Definition Fda.
From www.youtube.com
Calibration What is calibration? Definition of calibration Calibration Definition Fda calibration requirements for equipment are defined by title 21 of the fda’s code of federal regulations. Concomitant material refer to those. (a) there shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug. once drug development reaches the stage where the api is produced for use in. Calibration Definition Fda.
From www.slideserve.com
PPT Computer Simulation in Medical Diagnostic Assay Calibration Calibration Definition Fda § 58.63 maintenance and calibration of equipment. once drug development reaches the stage where the api is produced for use in drug products intended for. (a) there shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug. calibration requirements for equipment are defined by title 21. Calibration Definition Fda.
From www.slideshare.net
Instrument Calibration Calibration Definition Fda The cgmp regulations for validating pharmaceutical. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. calibration requirements for equipment are defined by title 21 of the fda’s code of federal regulations. Calibration procedures shall include specific directions and limits for accuracy and precision. Calibration procedures shall include specific directions and limits for. Calibration Definition Fda.
From www.studypool.com
SOLUTION Calibration definition types formulas example problems Calibration Definition Fda fda amended the definition of manufacturing material to help clarify this definition. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. calibration requirements for equipment are defined by title 21 of the fda’s code of federal regulations. Calibration procedures shall include specific directions and limits for accuracy and precision. The cgmp. Calibration Definition Fda.
From www.aifurnaces.com
What is Calibration & Why temperature calibration is important for lab Calibration Definition Fda calibration requirements for equipment are defined by title 21 of the fda’s code of federal regulations. Calibration procedures shall include specific directions and limits for accuracy and precision. The cgmp regulations for validating pharmaceutical. once drug development reaches the stage where the api is produced for use in drug products intended for. (a) equipment shall be adequately inspected,. Calibration Definition Fda.
From www.pharmaceuticalsky.com
Differences between Calibration, Verification and Validation Calibration Definition Fda fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. calibration requirements for equipment are defined by title 21 of the fda’s code of federal regulations. once drug development reaches the stage where the api is produced for use in drug products intended for. Calibration procedures shall include specific directions and limits. Calibration Definition Fda.
From ciqa.net
When is Necessary an Instrument Calibration as per FDA requirements? Calibration Definition Fda Calibration procedures shall include specific directions and limits for accuracy and precision. (a) there shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. once drug development reaches the stage where the. Calibration Definition Fda.
From dxonmvgqw.blob.core.windows.net
Calibration Definition In Analytical Chemistry at Victor Patton blog Calibration Definition Fda fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. Calibration procedures shall include specific directions and limits for accuracy and precision. The cgmp regulations for validating pharmaceutical. fda amended the definition of manufacturing material to help clarify this definition. once drug development reaches the stage where the api is produced for. Calibration Definition Fda.
From www.slideserve.com
PPT A Calibration and Validation Process (CAVP) for Complex Adaptive Calibration Definition Fda fda amended the definition of manufacturing material to help clarify this definition. Concomitant material refer to those. The cgmp regulations for validating pharmaceutical. once drug development reaches the stage where the api is produced for use in drug products intended for. (a) equipment shall be adequately inspected, cleaned, and. (a) there shall be a quality control unit. Calibration Definition Fda.
From www.vrogue.co
A Comprehensive Guide To Different Instrument Calibra vrogue.co Calibration Definition Fda Calibration procedures shall include specific directions and limits for accuracy and precision. (a) equipment shall be adequately inspected, cleaned, and. The cgmp regulations for validating pharmaceutical. fda amended the definition of manufacturing material to help clarify this definition. once drug development reaches the stage where the api is produced for use in drug products intended for. Calibration procedures. Calibration Definition Fda.
From www.youtube.com
The Calibration Process in the Pharmaceutical Industry YouTube Calibration Definition Fda (a) equipment shall be adequately inspected, cleaned, and. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. once drug development reaches the stage where the api is produced for use in drug products intended for. Calibration procedures shall include specific directions and limits for accuracy and precision. (a) there shall be. Calibration Definition Fda.
From www.youtube.com
Calibration Types Definition, Uses, Equipment, & Examples [Detailed Calibration Definition Fda The cgmp regulations for validating pharmaceutical. calibration requirements for equipment are defined by title 21 of the fda’s code of federal regulations. Calibration procedures shall include specific directions and limits for accuracy and precision. (a) there shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug. Concomitant material. Calibration Definition Fda.
From dxobzjaym.blob.core.windows.net
Calibration Definition In Healthcare at William Swindle blog Calibration Definition Fda fda amended the definition of manufacturing material to help clarify this definition. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. calibration requirements for equipment are defined by title 21 of the fda’s code of federal regulations. Calibration procedures shall include specific directions and limits for accuracy and precision. §. Calibration Definition Fda.
From dxoatrmoc.blob.core.windows.net
Calibration And Quality Control In Laboratory Pdf at Marcia Snyder blog Calibration Definition Fda § 58.63 maintenance and calibration of equipment. fda amended the definition of manufacturing material to help clarify this definition. Calibration procedures shall include specific directions and limits for accuracy and precision. (a) equipment shall be adequately inspected, cleaned, and. The cgmp regulations for validating pharmaceutical. Calibration procedures shall include specific directions and limits for accuracy and precision. . Calibration Definition Fda.
From exogfedck.blob.core.windows.net
What Is Image Calibration at Eleanor Albert blog Calibration Definition Fda once drug development reaches the stage where the api is produced for use in drug products intended for. Calibration procedures shall include specific directions and limits for accuracy and precision. fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. (a) there shall be a quality control unit that shall have the. Calibration Definition Fda.
From www.youtube.com
Basic Principles of Instrument Calibration YouTube Calibration Definition Fda Calibration procedures shall include specific directions and limits for accuracy and precision. (a) there shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug. Concomitant material refer to those. The cgmp regulations for validating pharmaceutical. fda has the authority and responsibility to inspect and evaluate process validation performed. Calibration Definition Fda.
From www.youtube.com
Introduction to Calibration Definition, types, purpose, Procedure of Calibration Definition Fda (a) equipment shall be adequately inspected, cleaned, and. § 58.63 maintenance and calibration of equipment. Concomitant material refer to those. Calibration procedures shall include specific directions and limits for accuracy and precision. Calibration procedures shall include specific directions and limits for accuracy and precision. fda has the authority and responsibility to inspect and evaluate process validation performed by. Calibration Definition Fda.
From www.studypool.com
SOLUTION Calibration definition types formulas example problems Calibration Definition Fda (a) equipment shall be adequately inspected, cleaned, and. fda amended the definition of manufacturing material to help clarify this definition. (a) there shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug. The cgmp regulations for validating pharmaceutical. fda has the authority and responsibility to inspect and. Calibration Definition Fda.
From www.slideserve.com
PPT INDUSTRIAL HYGIENE PRINCIPLES AND INSTRUMENTATION FOR Calibration Definition Fda calibration requirements for equipment are defined by title 21 of the fda’s code of federal regulations. (a) equipment shall be adequately inspected, cleaned, and. § 58.63 maintenance and calibration of equipment. Calibration procedures shall include specific directions and limits for accuracy and precision. fda has the authority and responsibility to inspect and evaluate process validation performed by. Calibration Definition Fda.
From www.slideshare.net
Calibration Calibration Definition Fda Calibration procedures shall include specific directions and limits for accuracy and precision. (a) there shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug. once drug development reaches the stage where the api is produced for use in drug products intended for. § 58.63 maintenance and calibration. Calibration Definition Fda.