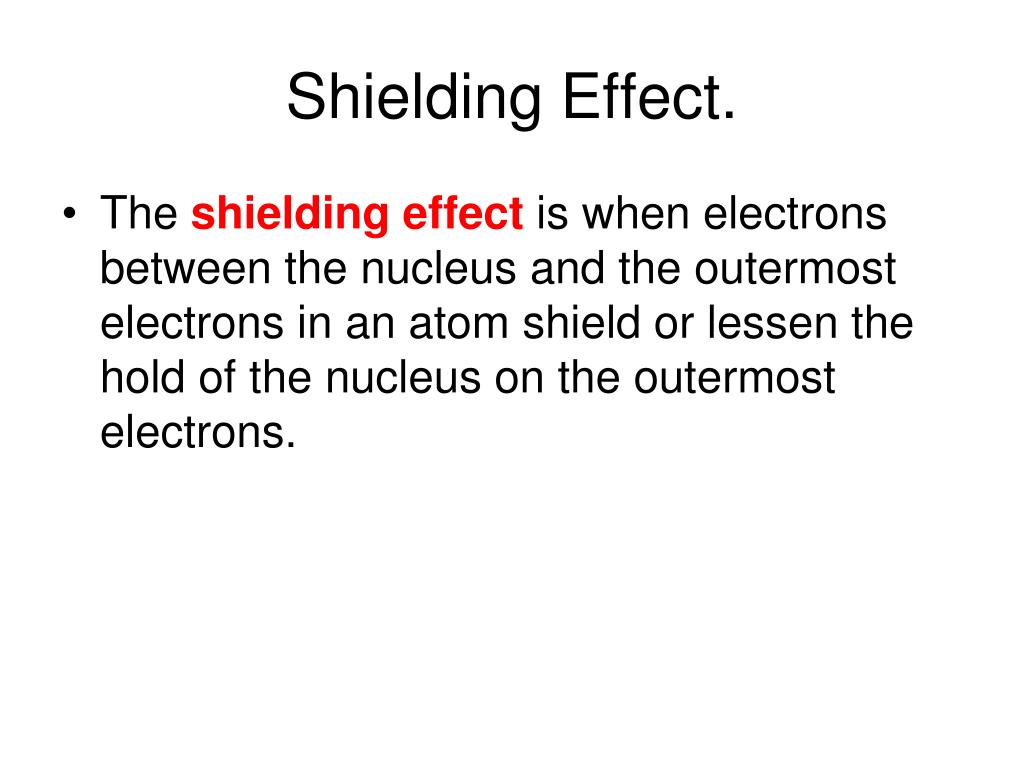
Define The Shielding Effect . the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. what is electron shielding. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. shielding effect : electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. when shielding is poor, the positively charged nucleus has a greater attraction to the electrons, and atomic radius decreases more.
from www.slideserve.com
shielding effect : what is electron shielding. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. when shielding is poor, the positively charged nucleus has a greater attraction to the electrons, and atomic radius decreases more. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner.
PPT Chapter 6 PowerPoint Presentation, free download ID4499242
Define The Shielding Effect when shielding is poor, the positively charged nucleus has a greater attraction to the electrons, and atomic radius decreases more. shielding effect : when shielding is poor, the positively charged nucleus has a greater attraction to the electrons, and atomic radius decreases more. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. what is electron shielding. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of.
From www.youtube.com
SHIELDING/SCREENING EFFECT YouTube Define The Shielding Effect The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and. Define The Shielding Effect.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting Define The Shielding Effect when shielding is poor, the positively charged nucleus has a greater attraction to the electrons, and atomic radius decreases more. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge.. Define The Shielding Effect.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID4499242 Define The Shielding Effect In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. what is electron shielding. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due. Define The Shielding Effect.
From byjus.com
What is shielding and deshielding in NMR? Give an example? Define The Shielding Effect the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. what is electron shielding. electron shielding refers to the blocking of valence shell electron attraction by. Define The Shielding Effect.
From www.youtube.com
Shielding and Deshielding H NMR Spectroscopy YouTube Define The Shielding Effect what is electron shielding. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. shielding effect : electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. when shielding is poor, the positively charged nucleus has. Define The Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons Define The Shielding Effect shielding effect : the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. In the realm of atomic structure, the concepts of slater’s rule, screening. Define The Shielding Effect.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID4499242 Define The Shielding Effect what is electron shielding. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. when shielding is poor, the positively charged nucleus has a. Define The Shielding Effect.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting Define The Shielding Effect what is electron shielding. when shielding is poor, the positively charged nucleus has a greater attraction to the electrons, and atomic radius decreases more. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The shielding effect describes the balance between the pull of. Define The Shielding Effect.
From www.youtube.com
418 Shielding Effect YouTube Define The Shielding Effect shielding effect : electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. what is electron shielding. In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. The shielding effect describes the balance between the pull of the protons on. Define The Shielding Effect.
From exoeftfzx.blob.core.windows.net
What Is Shielding Chem at Victor Riggs blog Define The Shielding Effect shielding effect : what is electron shielding. In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. electron shielding refers to the blocking of valence shell electron attraction. Define The Shielding Effect.
From socratic.org
How are shielding effect and atomic radius related? Socratic Define The Shielding Effect when shielding is poor, the positively charged nucleus has a greater attraction to the electrons, and atomic radius decreases more. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. . Define The Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons Define The Shielding Effect when shielding is poor, the positively charged nucleus has a greater attraction to the electrons, and atomic radius decreases more. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. electron shielding refers to the blocking of valence shell electron attraction by the nucleus,. Define The Shielding Effect.
From exozhcqdr.blob.core.windows.net
What Is Shielding Effect Simple Definition at Dawn Cook blog Define The Shielding Effect what is electron shielding. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. when shielding is poor, the positively charged nucleus has a greater attraction to. Define The Shielding Effect.
From animalia-life.club
Shielding Effect Trend Define The Shielding Effect electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. shielding effect : In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion. Define The Shielding Effect.
From exowuasqn.blob.core.windows.net
What Is Shielding Effect For Class 9 at Loretta Bryan blog Define The Shielding Effect shielding effect : electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. what is electron shielding. In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. when shielding is poor, the positively charged nucleus has a greater attraction. Define The Shielding Effect.
From mungfali.com
What Is Shielding Effect Define The Shielding Effect what is electron shielding. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. shielding effect : the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. when shielding is poor, the. Define The Shielding Effect.
From thechemistrynotes.com
Shielding effect Define The Shielding Effect the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. when shielding is poor, the positively charged nucleus has a greater attraction to the electrons, and atomic radius decreases more. shielding effect : electron shielding refers to the blocking of valence shell electron. Define The Shielding Effect.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity Define The Shielding Effect when shielding is poor, the positively charged nucleus has a greater attraction to the electrons, and atomic radius decreases more. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. shielding effect : the concept of electron shielding, in which intervening electrons act to reduce the. Define The Shielding Effect.
From www.youtube.com
Shielding Effect YouTube Define The Shielding Effect when shielding is poor, the positively charged nucleus has a greater attraction to the electrons, and atomic radius decreases more. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge.. Define The Shielding Effect.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The Define The Shielding Effect electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. when shielding is poor, the positively charged nucleus has a greater attraction to the electrons, and atomic radius decreases more. what is electron shielding. shielding effect : In the realm of atomic structure, the concepts of slater’s. Define The Shielding Effect.
From www.chem.fsu.edu
Electron Configurations Define The Shielding Effect electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. when shielding is poor, the positively charged nucleus has a greater attraction to the electrons, and atomic radius decreases more. shielding effect : the concept of electron shielding, in which intervening electrons act to reduce the positive. Define The Shielding Effect.
From animalia-life.club
Shielding Effect Trend Define The Shielding Effect electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. shielding effect : In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced. Define The Shielding Effect.
From chemisfast.blogspot.com
Lanthanide contractiondefinitioncausesconsequences in chemistry PG Define The Shielding Effect The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. when shielding is poor, the positively charged nucleus has a greater attraction to the electrons, and atomic radius decreases more. In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge.. Define The Shielding Effect.
From www.youtube.com
Trend of Shielding Effect in Periodic Table, Chemistry Lecture Sabaq Define The Shielding Effect when shielding is poor, the positively charged nucleus has a greater attraction to the electrons, and atomic radius decreases more. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The shielding effect describes the balance between the pull of the protons on valence electrons. Define The Shielding Effect.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 Define The Shielding Effect In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion. Define The Shielding Effect.
From www.youtube.com
Shielding Effect with Trend YouTube Define The Shielding Effect The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. what is electron shielding. In the realm of atomic structure, the concepts of slater’s. Define The Shielding Effect.
From www.mecamagnetic.com
All about shielding MECA Define The Shielding Effect what is electron shielding. In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. when shielding is poor, the positively charged nucleus has a greater attraction to the electrons, and atomic radius decreases more. The shielding effect describes the balance between the pull of the protons on valence electrons and. Define The Shielding Effect.
From www.wisegeek.com
What Is the Shielding Effect? (with pictures) Define The Shielding Effect shielding effect : electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. what is electron shielding. when shielding is poor, the positively charged nucleus has a greater attraction. Define The Shielding Effect.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube Define The Shielding Effect what is electron shielding. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. In the realm of atomic structure, the concepts of slater’s rule,. Define The Shielding Effect.
From www.youtube.com
Shielding effect or Screening effect YouTube Define The Shielding Effect what is electron shielding. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. shielding effect : The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. In the realm of atomic structure,. Define The Shielding Effect.
From exozhcqdr.blob.core.windows.net
What Is Shielding Effect Simple Definition at Dawn Cook blog Define The Shielding Effect electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. shielding effect : The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. when shielding is poor, the positively charged nucleus has a greater attraction to the. Define The Shielding Effect.
From localrevive.com
What Is It? How Does It Work? Materials (2022) Define The Shielding Effect the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. what is electron shielding. shielding effect : In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. when shielding is poor, the positively charged nucleus. Define The Shielding Effect.
From animalia-life.club
Shielding Effect Trend Define The Shielding Effect In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. what is electron shielding. when shielding is poor, the positively charged nucleus has a greater attraction. Define The Shielding Effect.
From exozhcqdr.blob.core.windows.net
What Is Shielding Effect Simple Definition at Dawn Cook blog Define The Shielding Effect the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. shielding effect : when shielding is poor, the positively charged nucleus has a. Define The Shielding Effect.
From www.vedantu.com
Shielding Effect and Effective Nuclear Charge Important Concepts for JEE Define The Shielding Effect the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. In the realm of atomic structure, the concepts of slater’s rule, screening effect (σ), and effective nuclear charge. what is electron shielding. when shielding is poor, the positively charged nucleus has a greater attraction. Define The Shielding Effect.