Chlorine Group Properties . Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is the second halogen in the periodic table, being a nonmetal in group 17. Chlorine is a member of the halogen family. Chlorine is a member of. Chlorine is a halogen in group 17 and period 3. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. It is very reactive and is widely used for many purposes, such as as a disinfectant. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher than that of fluorine. Hence, its properties are similar to.
from www.alamy.com
Chlorine is a halogen in group 17 and period 3. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher than that of fluorine. Chlorine is the second halogen in the periodic table, being a nonmetal in group 17. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It is very reactive and is widely used for many purposes, such as as a disinfectant. Hence, its properties are similar to. Chlorine is a member of. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Chlorine is a member of the halogen family.
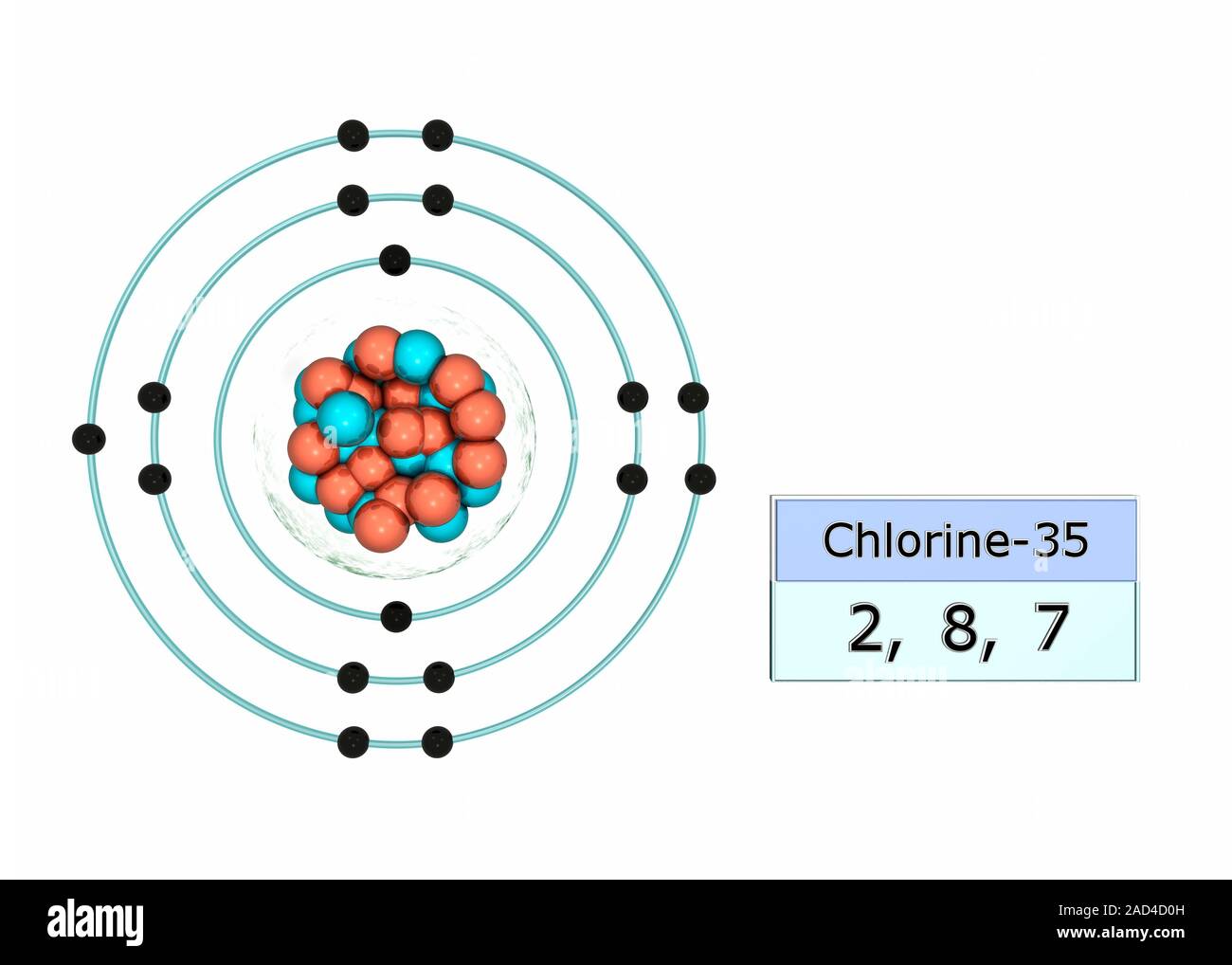
Chlorine electron configuration. Illustration of the atomic structure
Chlorine Group Properties Chlorine is a member of. Chlorine is a member of. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher than that of fluorine. Chlorine is a member of the halogen family. Hence, its properties are similar to. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It is very reactive and is widely used for many purposes, such as as a disinfectant. Chlorine is a halogen in group 17 and period 3. Chlorine is the second halogen in the periodic table, being a nonmetal in group 17.
From www.vedantu.com
Chlorine Learn Definition, Properties and Facts Chlorine Group Properties Chlorine is a member of. Chlorine is a halogen in group 17 and period 3. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher. Chlorine Group Properties.
From studiousguy.com
Chlorine (Cl) Properties & Uses StudiousGuy Chlorine Group Properties Chlorine is the second halogen in the periodic table, being a nonmetal in group 17. Chlorine is a member of. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is a member of the halogen family. Chlorine is a. Chlorine Group Properties.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Group Properties Chlorine is the second halogen in the periodic table, being a nonmetal in group 17. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher than that of fluorine. Chlorine is a halogen in group 17 and period 3. Chlorine is a member of. Halogens are the elements that make up group 17 (viia). Chlorine Group Properties.
From pediaa.com
Difference Between Chlorine and Chloride Definition, Properties Chlorine Group Properties Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is a member of. Chlorine is a member of the halogen family. Hence, its properties are similar to. It is very reactive and is widely used for many purposes, such as as a disinfectant. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher than. Chlorine Group Properties.
From www.slideserve.com
PPT Chlorine PowerPoint Presentation, free download ID2277012 Chlorine Group Properties Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is a halogen in group 17 and period 3. It is very reactive and is widely used for many purposes, such as as a disinfectant. Hence, its properties are similar. Chlorine Group Properties.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun Chlorine Group Properties Chlorine is the second halogen in the periodic table, being a nonmetal in group 17. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Chlorine is a halogen in group 17 and period 3. It is very reactive and is widely used for many purposes, such as as. Chlorine Group Properties.
From elchoroukhost.net
Chlorine Periodic Table Properties Elcho Table Chlorine Group Properties Chlorine is a halogen in group 17 and period 3. Chlorine is the second halogen in the periodic table, being a nonmetal in group 17. Chlorine is a member of. Chlorine is a member of the halogen family. Hence, its properties are similar to. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher. Chlorine Group Properties.
From www.istockphoto.com
Cl Chlorine Element Information Facts Properties Trends Uses And Chlorine Group Properties Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is the second halogen in the periodic table, being a nonmetal in group 17. It is very reactive and is widely used for many purposes, such as as a disinfectant. Chlorine is a halogen in group 17 and period 3. Chlorine has a high electronegativity and a high electron affinity, the. Chlorine Group Properties.
From periodictableguide.com
Chlorine (Cl) Periodic Table (Element Information & More) Chlorine Group Properties Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher than that of fluorine. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Hence, its properties are similar to. Chlorine is a member of. Chlorine is the second halogen in the periodic table, being a nonmetal in group 17. Chlorine is a halogen in group. Chlorine Group Properties.
From www.examples.com
Chlorine (Cl) Definition, Preparation, Properties, Uses, Compounds Chlorine Group Properties Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. It is very reactive and is widely used for many purposes, such as as a disinfectant. Chlorine is a member of. Hence, its properties are similar to. Chlorine has a high electronegativity and a high electron affinity, the latter. Chlorine Group Properties.
From www.youtube.com
Chlorine Gas Physical Properties YouTube Chlorine Group Properties Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is a member of the halogen family. It is very reactive and is widely used for many purposes, such as as a disinfectant. Chlorine is a member of. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher than that of fluorine. Halogens are the. Chlorine Group Properties.
From eduinput.com
ChlorineDiscovery, Properties, And Applications Chlorine Group Properties It is very reactive and is widely used for many purposes, such as as a disinfectant. Chlorine is a member of. Chlorine is the second halogen in the periodic table, being a nonmetal in group 17. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Chlorine is a. Chlorine Group Properties.
From melscience.com
Characteristics and properties of chlorine, and its reactions with Chlorine Group Properties Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Hence, its properties are similar to. Chlorine is the second halogen in the periodic table, being a nonmetal in group 17. It is very reactive and is widely used for many purposes, such as as a disinfectant. Chlorine is. Chlorine Group Properties.
From www.examples.com
Chlorine (Cl) Definition, Preparation, Properties, Uses, Compounds Chlorine Group Properties Chlorine is the second halogen in the periodic table, being a nonmetal in group 17. Chlorine is a halogen in group 17 and period 3. Hence, its properties are similar to. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher than that of fluorine. Halogens are the elements that make up group 17. Chlorine Group Properties.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Group Properties Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It is very reactive and is widely used for many purposes, such as as a disinfectant. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly. Chlorine Group Properties.
From www.dreamstime.com
Chlorine Periodic Table of the Elements Vector Stock Vector Chlorine Group Properties Chlorine is a halogen in group 17 and period 3. Hence, its properties are similar to. Chlorine is a member of the halogen family. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Chlorine is the second halogen in the periodic table, being a nonmetal in group 17.. Chlorine Group Properties.
From www.alamy.com
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock Chlorine Group Properties It is very reactive and is widely used for many purposes, such as as a disinfectant. Chlorine is a member of. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher than that. Chlorine Group Properties.
From winter.group.shef.ac.uk
Elements Periodic Table » Chlorine » properties of free atoms Chlorine Group Properties Chlorine is a member of. Chlorine is a halogen in group 17 and period 3. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. It is very reactive and is widely used for many purposes, such as as a disinfectant. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,.. Chlorine Group Properties.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Chlorine Group Properties Hence, its properties are similar to. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher than that of fluorine. Chlorine is a member of the halogen family. Chlorine is a member of. It is very reactive and is widely used for many purposes, such as as a disinfectant. Halogens are the elements that. Chlorine Group Properties.
From material-properties.org
Sodium and Chlorine Comparison Properties Material Properties Chlorine Group Properties Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is a member of. Chlorine is the second halogen in the periodic table, being a nonmetal in group 17. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Hence, its properties are similar to. It is very reactive and. Chlorine Group Properties.
From www.britannica.com
chlorine Uses, Properties, & Facts Britannica Chlorine Group Properties Chlorine is a member of. Chlorine is a member of the halogen family. Chlorine is a halogen in group 17 and period 3. Hence, its properties are similar to. It is very reactive and is widely used for many purposes, such as as a disinfectant. Chlorine is the second halogen in the periodic table, being a nonmetal in group 17.. Chlorine Group Properties.
From www.theowletscience.org
Chemistry The Periodic Table of the Elements. Chlorine the owlet Chlorine Group Properties Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is a member of the halogen family. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. It is very reactive and is widely used for many purposes, such as as a disinfectant. Chlorine is a member of. Chlorine has. Chlorine Group Properties.
From studiousguy.com
Chlorine (Cl) Properties & Uses StudiousGuy Chlorine Group Properties It is very reactive and is widely used for many purposes, such as as a disinfectant. Chlorine is a member of the halogen family. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly. Chlorine Group Properties.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure Chlorine Group Properties Hence, its properties are similar to. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is a member of. Chlorine is a member of the halogen family. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher than that of fluorine. It is very reactive and is widely used for many purposes, such as. Chlorine Group Properties.
From www.slideserve.com
PPT Chlorine PowerPoint Presentation, free download ID2277012 Chlorine Group Properties Chlorine is a member of the halogen family. Hence, its properties are similar to. Chlorine is the second halogen in the periodic table, being a nonmetal in group 17. Chlorine is a halogen in group 17 and period 3. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Halogens are the elements that make up group 17 (viia) of the periodic. Chlorine Group Properties.
From www.slideserve.com
PPT Chapter 4 Formation of Compounds PowerPoint Presentation, free Chlorine Group Properties Hence, its properties are similar to. Chlorine is the second halogen in the periodic table, being a nonmetal in group 17. It is very reactive and is widely used for many purposes, such as as a disinfectant. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Chlorine is. Chlorine Group Properties.
From www.americanelements.com
Chlorine (Cl) AMERICAN ELEMENTS Chlorine Group Properties Chlorine is a member of the halogen family. Chlorine is a halogen in group 17 and period 3. It is very reactive and is widely used for many purposes, such as as a disinfectant. Hence, its properties are similar to. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Halogens are the elements that make up group 17 (viia) of the. Chlorine Group Properties.
From www.animalia-life.club
Chlorine Element Periodic Table Chlorine Group Properties Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher than that of fluorine. Chlorine is the second halogen in the periodic table, being a nonmetal in group 17. Chlorine is a member of the halogen family. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that. Chlorine Group Properties.
From www.slideserve.com
PPT Chlorine ( Cl ) PowerPoint Presentation, free download ID2276942 Chlorine Group Properties Chlorine is a member of the halogen family. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is a member of. Hence, its properties are similar to. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Chlorine has a high electronegativity and a high electron affinity, the latter. Chlorine Group Properties.
From www.examples.com
Chlorine (Cl) Definition, Preparation, Properties, Uses, Compounds Chlorine Group Properties Chlorine is a halogen in group 17 and period 3. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. It is very reactive and is widely used for many purposes, such as as a disinfectant. Hence, its properties are similar to. Chlorine is a member of. Sources, facts,. Chlorine Group Properties.
From www.youtube.com
Chemical Properties of Chlorine Reactions of Chlorine YouTube Chlorine Group Properties Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is a member of. Chlorine is a halogen in group 17 and period 3. Hence, its properties are similar to. Chlorine is a member of the halogen family. It is very reactive and is widely used for many purposes, such as as a disinfectant. Chlorine is the second halogen in the. Chlorine Group Properties.
From elchoroukhost.net
Chlorine Periodic Table Properties Elcho Table Chlorine Group Properties Hence, its properties are similar to. It is very reactive and is widely used for many purposes, such as as a disinfectant. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher than. Chlorine Group Properties.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun Chlorine Group Properties Hence, its properties are similar to. Chlorine is a member of the halogen family. Chlorine is a halogen in group 17 and period 3. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. It is very reactive and is widely used for many purposes, such as as a. Chlorine Group Properties.
From www.nuclear-power.com
What is Chlorine Properties of Chlorine Element Symbol Cl nuclear Chlorine Group Properties Chlorine is a halogen in group 17 and period 3. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher than that of fluorine. Halogens are the elements that make up group 17 (viia) of the periodic table, a chart that shows how elements are. Chlorine. Chlorine Group Properties.
From chemicalengineeringworld.com
Chlorine Element Properties Archives Chemical Engineering World Chlorine Group Properties Hence, its properties are similar to. Chlorine is a member of the halogen family. It is very reactive and is widely used for many purposes, such as as a disinfectant. Chlorine has a high electronegativity and a high electron affinity, the latter being even slightly higher than that of fluorine. Chlorine is the second halogen in the periodic table, being. Chlorine Group Properties.