Lab 8 Questions For Heats Of Reaction . Study with quizlet and memorize flashcards containing terms like specific heat, intensive property, the zeroth law of thermodynamics and. Observe an endothermic reaction 3. Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. Learn how to measure the heat of reaction and the specific heat of a substance using a calorimeter. Heats of reaction with extension. Understand the heat of reactions 2. Heats of reaction with extension goals 1. Observe an endothermic reaction 3. Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. Understand the heat of reactions 2. The enthalpy of a reaction is the amount of energy or heat absorbed or released into a reaction. If energy is required, this is a positive. The amount of heat gained or lost during a reaction is due to the breaking of bonds between atoms in the reactants and the formation of.
from www.chegg.com
Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. Study with quizlet and memorize flashcards containing terms like specific heat, intensive property, the zeroth law of thermodynamics and. Understand the heat of reactions 2. Understand the heat of reactions 2. If energy is required, this is a positive. Heats of reaction with extension. The amount of heat gained or lost during a reaction is due to the breaking of bonds between atoms in the reactants and the formation of. Observe an endothermic reaction 3. Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. Learn how to measure the heat of reaction and the specific heat of a substance using a calorimeter.
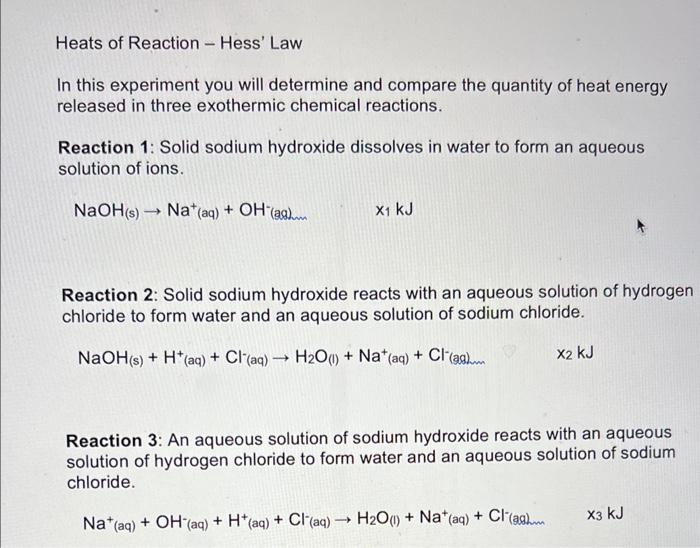
Solved Heats of Reaction Hess' Law In this experiment you
Lab 8 Questions For Heats Of Reaction Understand the heat of reactions 2. Heats of reaction with extension goals 1. If energy is required, this is a positive. Learn how to measure the heat of reaction and the specific heat of a substance using a calorimeter. Observe an endothermic reaction 3. The amount of heat gained or lost during a reaction is due to the breaking of bonds between atoms in the reactants and the formation of. Heats of reaction with extension. Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. Observe an endothermic reaction 3. Study with quizlet and memorize flashcards containing terms like specific heat, intensive property, the zeroth law of thermodynamics and. Understand the heat of reactions 2. The enthalpy of a reaction is the amount of energy or heat absorbed or released into a reaction. Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. Understand the heat of reactions 2.
From www.flinnsci.com
Heats of Reaction and Hess’s Law SmallScale Calorimetry—ChemTopic Lab 8 Questions For Heats Of Reaction Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. Study with quizlet and memorize flashcards containing terms like specific heat, intensive property, the zeroth law of thermodynamics and. The amount of heat gained or lost during a reaction is due to the breaking of bonds between atoms in the reactants and the. Lab 8 Questions For Heats Of Reaction.
From www.studocu.com
Lab Hess' Law Simulation Heats of Reaction Hess' Law Heats of Lab 8 Questions For Heats Of Reaction Observe an endothermic reaction 3. Understand the heat of reactions 2. Heats of reaction with extension. The amount of heat gained or lost during a reaction is due to the breaking of bonds between atoms in the reactants and the formation of. Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. The. Lab 8 Questions For Heats Of Reaction.
From www.chegg.com
Solved HEATS OF REACTIONS II Answer the following and give Lab 8 Questions For Heats Of Reaction Study with quizlet and memorize flashcards containing terms like specific heat, intensive property, the zeroth law of thermodynamics and. Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. Observe an endothermic reaction 3. Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. Heats of. Lab 8 Questions For Heats Of Reaction.
From www.scribd.com
Additivity of Heats of Reaction Hess’s Law Hydroxide Aqueous Solution Lab 8 Questions For Heats Of Reaction Learn how to measure the heat of reaction and the specific heat of a substance using a calorimeter. The amount of heat gained or lost during a reaction is due to the breaking of bonds between atoms in the reactants and the formation of. Find out how to correct for the heat capacity of the calorimeter and the surroundings, and. Lab 8 Questions For Heats Of Reaction.
From www.chegg.com
Solved Additivity of Heats of Reaction Hess's Law In this Lab 8 Questions For Heats Of Reaction Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. Understand the heat of reactions 2. The amount of heat gained or lost during a reaction is due to the breaking of bonds between atoms in the reactants and the formation of. The enthalpy of a reaction is the amount of energy or. Lab 8 Questions For Heats Of Reaction.
From www.chegg.com
Solved Heats of Reaction Lab (Hess' Law) Purpose To Lab 8 Questions For Heats Of Reaction Learn how to measure the heat of reaction and the specific heat of a substance using a calorimeter. Observe an endothermic reaction 3. Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. Understand the heat of reactions 2. If energy is required, this is a positive. Heats of reaction with extension. Find. Lab 8 Questions For Heats Of Reaction.
From studylib.net
Types of Reactions Using Heat Lab Lab 8 Questions For Heats Of Reaction Learn how to measure the heat of reaction and the specific heat of a substance using a calorimeter. Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. The enthalpy of a reaction is the amount. Lab 8 Questions For Heats Of Reaction.
From www.chegg.com
Solved Lab 07 Specific Heats of Materials and Reactions The Lab 8 Questions For Heats Of Reaction Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. Heats of reaction with extension. Observe an endothermic reaction 3. Observe an endothermic reaction 3. Understand the heat of reactions 2. Study with quizlet and memorize. Lab 8 Questions For Heats Of Reaction.
From www.chegg.com
Solved Experiment 18 Additivity of Heats of Reaction Hess's Lab 8 Questions For Heats Of Reaction Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. Observe an endothermic reaction 3. Understand the heat of reactions 2. The amount of heat gained or lost during a reaction is due to the breaking of bonds between atoms in the reactants and the formation of. Heats of reaction with extension. Observe. Lab 8 Questions For Heats Of Reaction.
From www.chegg.com
Experiment 18 Additivity of Heats of Reaction Hess' Lab 8 Questions For Heats Of Reaction Heats of reaction with extension. Understand the heat of reactions 2. Learn how to measure the heat of reaction and the specific heat of a substance using a calorimeter. The enthalpy of a reaction is the amount of energy or heat absorbed or released into a reaction. Observe an endothermic reaction 3. Understand the heat of reactions 2. Observe an. Lab 8 Questions For Heats Of Reaction.
From www.scribd.com
Heats of Reactions LAB PDF Sodium Hydroxide Mole (Unit) Lab 8 Questions For Heats Of Reaction Heats of reaction with extension. Understand the heat of reactions 2. Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. The enthalpy of a reaction is the amount of energy or heat absorbed or released. Lab 8 Questions For Heats Of Reaction.
From www.studypool.com
SOLUTION Measuring Heats of Reactions Pre Lab Questions Studypool Lab 8 Questions For Heats Of Reaction Understand the heat of reactions 2. Study with quizlet and memorize flashcards containing terms like specific heat, intensive property, the zeroth law of thermodynamics and. Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. Understand the heat of reactions 2. If energy is required, this is a positive. Heats of reaction with. Lab 8 Questions For Heats Of Reaction.
From www.coursehero.com
9.4.3 Lab Heats of Reaction.pdf Heats of Reaction Semester 2 Unit 3 Lab 8 Questions For Heats Of Reaction Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. The enthalpy of a reaction is the amount of energy or heat absorbed or released into a reaction. Heats of reaction with extension goals 1. Study with quizlet and memorize flashcards containing terms like specific heat, intensive property, the zeroth law of thermodynamics. Lab 8 Questions For Heats Of Reaction.
From www.studocu.com
Heats of Reaction Hess' Law Lab Observation Table Reaction Mass of Lab 8 Questions For Heats Of Reaction Learn how to measure the heat of reaction and the specific heat of a substance using a calorimeter. The amount of heat gained or lost during a reaction is due to the breaking of bonds between atoms in the reactants and the formation of. Heats of reaction with extension goals 1. If energy is required, this is a positive. Understand. Lab 8 Questions For Heats Of Reaction.
From www.youtube.com
Experiment 8 Heats of Reaction SMU Chemistry YouTube Lab 8 Questions For Heats Of Reaction Observe an endothermic reaction 3. Observe an endothermic reaction 3. If energy is required, this is a positive. Heats of reaction with extension. The enthalpy of a reaction is the amount of energy or heat absorbed or released into a reaction. Understand the heat of reactions 2. Heats of reaction with extension goals 1. Understand the heat of reactions 2.. Lab 8 Questions For Heats Of Reaction.
From www.chegg.com
Heats of reaction and Hess’s law post lab 19 Lab 8 Questions For Heats Of Reaction The enthalpy of a reaction is the amount of energy or heat absorbed or released into a reaction. Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. Observe an endothermic reaction 3. Heats of reaction with extension goals 1. Observe an endothermic reaction 3. Learn how to measure the heat of reaction. Lab 8 Questions For Heats Of Reaction.
From www.chegg.com
Solved Experiment 9 Heats of Reaction PreLab Questions Lab 8 Questions For Heats Of Reaction The amount of heat gained or lost during a reaction is due to the breaking of bonds between atoms in the reactants and the formation of. Observe an endothermic reaction 3. Understand the heat of reactions 2. Heats of reaction with extension. Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. Study. Lab 8 Questions For Heats Of Reaction.
From exoyrbcod.blob.core.windows.net
Heats Of Reaction And Hess's Law Lab at Sam Edwards blog Lab 8 Questions For Heats Of Reaction Study with quizlet and memorize flashcards containing terms like specific heat, intensive property, the zeroth law of thermodynamics and. The enthalpy of a reaction is the amount of energy or heat absorbed or released into a reaction. Heats of reaction with extension. Understand the heat of reactions 2. If energy is required, this is a positive. Find out how to. Lab 8 Questions For Heats Of Reaction.
From www.studocu.com
Heat of a Reaction Lab Report Chem 1162 Heat of a Reaction Lab Lab 8 Questions For Heats Of Reaction Heats of reaction with extension goals 1. Study with quizlet and memorize flashcards containing terms like specific heat, intensive property, the zeroth law of thermodynamics and. Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. Heats of reaction with extension. Observe an endothermic reaction 3. The amount of heat gained or lost. Lab 8 Questions For Heats Of Reaction.
From answerfullwinslow.z14.web.core.windows.net
How To Determine Heat Of Reaction Lab 8 Questions For Heats Of Reaction Study with quizlet and memorize flashcards containing terms like specific heat, intensive property, the zeroth law of thermodynamics and. Understand the heat of reactions 2. Heats of reaction with extension. Observe an endothermic reaction 3. Observe an endothermic reaction 3. Heats of reaction with extension goals 1. The amount of heat gained or lost during a reaction is due to. Lab 8 Questions For Heats Of Reaction.
From www.chegg.com
Solved Additivity of Heats of Reaction Hess's Law Lab 8 Questions For Heats Of Reaction Learn how to measure the heat of reaction and the specific heat of a substance using a calorimeter. Heats of reaction with extension goals 1. Heats of reaction with extension. Understand the heat of reactions 2. Understand the heat of reactions 2. If energy is required, this is a positive. Find out how to correct for the heat capacity of. Lab 8 Questions For Heats Of Reaction.
From www.studypool.com
SOLUTION 3 3 hess s law using heats of formation to determine heats of Lab 8 Questions For Heats Of Reaction Observe an endothermic reaction 3. Understand the heat of reactions 2. Heats of reaction with extension goals 1. The enthalpy of a reaction is the amount of energy or heat absorbed or released into a reaction. Observe an endothermic reaction 3. Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. Enthalpy of. Lab 8 Questions For Heats Of Reaction.
From studylib.net
HEAT OF REACTION LAB Lab 8 Questions For Heats Of Reaction The enthalpy of a reaction is the amount of energy or heat absorbed or released into a reaction. Observe an endothermic reaction 3. Observe an endothermic reaction 3. Understand the heat of reactions 2. Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. The amount of heat gained or lost during a. Lab 8 Questions For Heats Of Reaction.
From www.studypool.com
SOLUTION 3 3 hess s law using heats of formation to determine heats of Lab 8 Questions For Heats Of Reaction The enthalpy of a reaction is the amount of energy or heat absorbed or released into a reaction. Heats of reaction with extension. Heats of reaction with extension goals 1. Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. Understand the heat of reactions 2. Observe an endothermic reaction 3. Study with. Lab 8 Questions For Heats Of Reaction.
From www.studocu.com
IC 4603 L07 Heats of Reactions PRELAB QUESTIONS 1. Water and steam Lab 8 Questions For Heats Of Reaction The enthalpy of a reaction is the amount of energy or heat absorbed or released into a reaction. Learn how to measure the heat of reaction and the specific heat of a substance using a calorimeter. Heats of reaction with extension goals 1. Observe an endothermic reaction 3. Observe an endothermic reaction 3. Find out how to correct for the. Lab 8 Questions For Heats Of Reaction.
From studylib.net
Heats of Reaction LabHess' Law Lab 8 Questions For Heats Of Reaction Understand the heat of reactions 2. Learn how to measure the heat of reaction and the specific heat of a substance using a calorimeter. Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. The amount of heat gained or lost during a reaction is due to the breaking of bonds between atoms. Lab 8 Questions For Heats Of Reaction.
From learncheme.com
heatsofreactionsummary LearnChemE Lab 8 Questions For Heats Of Reaction Study with quizlet and memorize flashcards containing terms like specific heat, intensive property, the zeroth law of thermodynamics and. Observe an endothermic reaction 3. The amount of heat gained or lost during a reaction is due to the breaking of bonds between atoms in the reactants and the formation of. Find out how to correct for the heat capacity of. Lab 8 Questions For Heats Of Reaction.
From www.chegg.com
Solved Measuring Heats of Reactions PRELAB QUESTIONS 1. Lab 8 Questions For Heats Of Reaction If energy is required, this is a positive. Heats of reaction with extension. Understand the heat of reactions 2. Observe an endothermic reaction 3. Study with quizlet and memorize flashcards containing terms like specific heat, intensive property, the zeroth law of thermodynamics and. Heats of reaction with extension goals 1. The amount of heat gained or lost during a reaction. Lab 8 Questions For Heats Of Reaction.
From www.youtube.com
Pre Lab Exp 2 Determining the heat of reaction Teacher Tang YouTube Lab 8 Questions For Heats Of Reaction Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. Learn how to measure the heat of reaction and the specific heat of a substance using a calorimeter. Observe an endothermic reaction 3. The enthalpy of a reaction is the amount of energy or heat absorbed or released into a reaction. If energy. Lab 8 Questions For Heats Of Reaction.
From www.studocu.com
Hess's Law assignment Experiment Additivity of Heats of Reaction Lab 8 Questions For Heats Of Reaction Observe an endothermic reaction 3. Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. Understand the heat of reactions 2. Heats of reaction with extension. Observe an endothermic reaction 3. Heats of reaction with extension goals 1. Enthalpy of a reaction is the amount of energy or heat absorbed or released in. Lab 8 Questions For Heats Of Reaction.
From www.chegg.com
Solved Heats of Reaction Hess' Law In this experiment you Lab 8 Questions For Heats Of Reaction Study with quizlet and memorize flashcards containing terms like specific heat, intensive property, the zeroth law of thermodynamics and. Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. The amount of heat gained or lost during a reaction is due to the breaking of bonds between atoms in the reactants and the. Lab 8 Questions For Heats Of Reaction.
From studylib.net
heats of reaction KEY Lab 8 Questions For Heats Of Reaction The amount of heat gained or lost during a reaction is due to the breaking of bonds between atoms in the reactants and the formation of. Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. Observe an endothermic reaction 3. Understand the heat of reactions 2. Observe an endothermic reaction 3. Study. Lab 8 Questions For Heats Of Reaction.
From www.chegg.com
Solved Additivity of Heats of Reaction Hess's Law In this Lab 8 Questions For Heats Of Reaction Heats of reaction with extension. If energy is required, this is a positive. The enthalpy of a reaction is the amount of energy or heat absorbed or released into a reaction. Understand the heat of reactions 2. Observe an endothermic reaction 3. Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. Study. Lab 8 Questions For Heats Of Reaction.
From www.scribd.com
Lab 8 Heats of Reaction PDF Sodium Bicarbonate Chemical Reactions Lab 8 Questions For Heats Of Reaction Find out how to correct for the heat capacity of the calorimeter and the surroundings, and how. Observe an endothermic reaction 3. Study with quizlet and memorize flashcards containing terms like specific heat, intensive property, the zeroth law of thermodynamics and. Understand the heat of reactions 2. Observe an endothermic reaction 3. The enthalpy of a reaction is the amount. Lab 8 Questions For Heats Of Reaction.
From www.chegg.com
Experiment 18 Additivity of Heats of Reaction Hess's Lab 8 Questions For Heats Of Reaction Enthalpy of a reaction is the amount of energy or heat absorbed or released in a reaction. Observe an endothermic reaction 3. Observe an endothermic reaction 3. Understand the heat of reactions 2. Understand the heat of reactions 2. Learn how to measure the heat of reaction and the specific heat of a substance using a calorimeter. Heats of reaction. Lab 8 Questions For Heats Of Reaction.