Reaction Between Zinc Oxide And Hydrochloric Acid Formula . This reaction is an example of a single displacement reaction, where one element replaces. 5.2 oxidation of zinc by hydrochloric acid subjects: During this reaction, the zinc metal reacts with the hydrochloric acid to produce zinc chloride and hydrogen gas. The reaction between zinc oxide (zno) and dilute. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. Metal oxides are generally basic in nature, therefore, zinc oxide will. Balanced equation for the reaction between zinc oxide and dilute hydrochloric acid: In the process, hydrogen gas is produced. Zinc oxide is represented by ${\text{zno}}$ and hydrochloric acid by ${\text{hcl}}$. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. Zinc is oxidized by hydrochloric acid to form zinc chloride. The reaction is given below.
from www.numerade.com
Zinc oxide is represented by ${\text{zno}}$ and hydrochloric acid by ${\text{hcl}}$. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. Metal oxides are generally basic in nature, therefore, zinc oxide will. During this reaction, the zinc metal reacts with the hydrochloric acid to produce zinc chloride and hydrogen gas. The reaction is given below. This reaction is an example of a single displacement reaction, where one element replaces. 5.2 oxidation of zinc by hydrochloric acid subjects: Zinc is oxidized by hydrochloric acid to form zinc chloride. The reaction between zinc oxide (zno) and dilute.
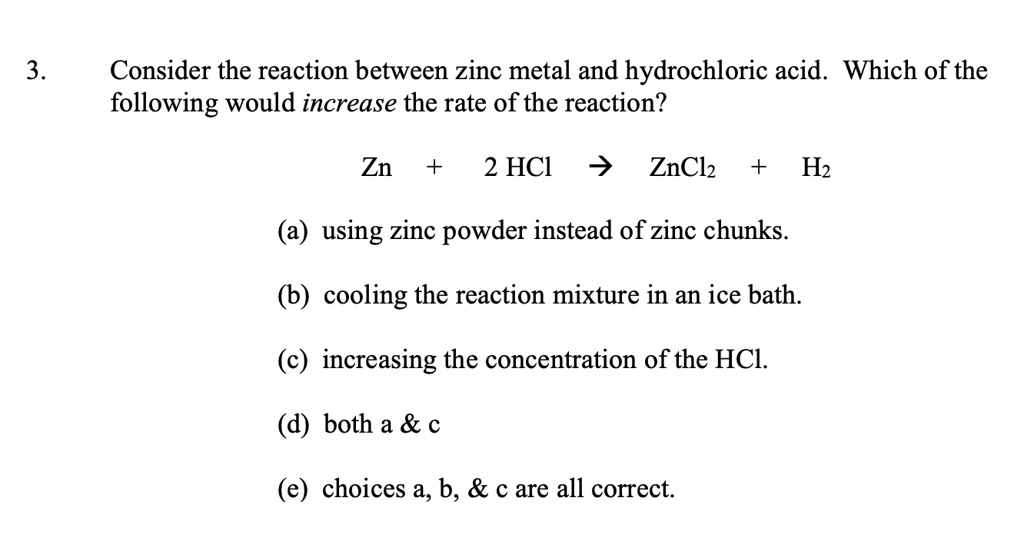
SOLVED Consider the reaction between zinc metal and hydrochloric acid
Reaction Between Zinc Oxide And Hydrochloric Acid Formula Metal oxides are generally basic in nature, therefore, zinc oxide will. During this reaction, the zinc metal reacts with the hydrochloric acid to produce zinc chloride and hydrogen gas. Zinc is oxidized by hydrochloric acid to form zinc chloride. Metal oxides are generally basic in nature, therefore, zinc oxide will. 5.2 oxidation of zinc by hydrochloric acid subjects: In the process, hydrogen gas is produced. The reaction is given below. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. Balanced equation for the reaction between zinc oxide and dilute hydrochloric acid: Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. This reaction is an example of a single displacement reaction, where one element replaces. The reaction between zinc oxide (zno) and dilute. Zinc oxide is represented by ${\text{zno}}$ and hydrochloric acid by ${\text{hcl}}$.
From brainly.in
What do you call the metal oxide having both acidic and basic Reaction Between Zinc Oxide And Hydrochloric Acid Formula Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. The reaction between zinc oxide (zno) and dilute. The reaction is given below. Zinc oxide is represented by ${\text{zno}}$ and hydrochloric acid by ${\text{hcl}}$. In the process, hydrogen gas is produced. Metal oxides. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Reaction Between Zinc Oxide And Hydrochloric Acid Formula The reaction between zinc oxide (zno) and dilute. This reaction is an example of a single displacement reaction, where one element replaces. Metal oxides are generally basic in nature, therefore, zinc oxide will. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. Balanced equation for the reaction between zinc oxide and dilute hydrochloric acid: Zinc is oxidized by. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.nagwa.com
Question Video Describing the Correct Symbol Equation for the Reaction Reaction Between Zinc Oxide And Hydrochloric Acid Formula Balanced equation for the reaction between zinc oxide and dilute hydrochloric acid: Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. 5.2 oxidation of zinc by hydrochloric acid subjects: Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. Zinc is. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From shotprofessional22.gitlab.io
Divine Zinc Plus Hydrochloric Acid Balanced Equation Different Formulas Reaction Between Zinc Oxide And Hydrochloric Acid Formula This reaction is an example of a single displacement reaction, where one element replaces. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. The reaction between zinc oxide (zno) and dilute. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions.. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.youtube.com
Identify hydrochloric acid and sulfuric acid solutions HCl and H2SO4 Reaction Between Zinc Oxide And Hydrochloric Acid Formula Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. 5.2 oxidation of zinc by hydrochloric acid subjects: The reaction between zinc oxide (zno) and dilute. In the process, hydrogen gas is produced. This reaction is an example of a single displacement reaction,. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From readingandwritingprojectcom.web.fc2.com
zinc metal and hydrochloric acid Reaction Between Zinc Oxide And Hydrochloric Acid Formula This reaction is an example of a single displacement reaction, where one element replaces. Balanced equation for the reaction between zinc oxide and dilute hydrochloric acid: Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. 5.2 oxidation of zinc by hydrochloric acid. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.nagwa.com
Question Video Identifying the Products When a Metal Oxide Reacts with Reaction Between Zinc Oxide And Hydrochloric Acid Formula Zinc is oxidized by hydrochloric acid to form zinc chloride. This reaction is an example of a single displacement reaction, where one element replaces. The reaction is given below. Zinc oxide is represented by ${\text{zno}}$ and hydrochloric acid by ${\text{hcl}}$. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. In the process, hydrogen gas is produced. Metal oxides. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube Reaction Between Zinc Oxide And Hydrochloric Acid Formula 5.2 oxidation of zinc by hydrochloric acid subjects: The reaction is given below. Balanced equation for the reaction between zinc oxide and dilute hydrochloric acid: Zinc is oxidized by hydrochloric acid to form zinc chloride. In the process, hydrogen gas is produced. Zinc oxide is represented by ${\text{zno}}$ and hydrochloric acid by ${\text{hcl}}$. Oxidation/reduction, gas forming reaction, acid properties, net. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Reaction Between Zinc Oxide And Hydrochloric Acid Formula Balanced equation for the reaction between zinc oxide and dilute hydrochloric acid: The reaction is given below. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. 5.2 oxidation of zinc by hydrochloric acid subjects: Metal oxides are generally basic in nature, therefore,. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Reaction Between Zinc Oxide And Hydrochloric Acid Formula Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. 5.2 oxidation of zinc by hydrochloric acid subjects: Metal oxides are generally basic in nature, therefore, zinc oxide will. Balanced equation for. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From tia-jolpblogramsey.blogspot.com
Zinc Carbonate Sulfuric Acid Reaction Between Zinc Oxide And Hydrochloric Acid Formula The reaction between zinc oxide (zno) and dilute. Zinc oxide is represented by ${\text{zno}}$ and hydrochloric acid by ${\text{hcl}}$. 5.2 oxidation of zinc by hydrochloric acid subjects: In the process, hydrogen gas is produced. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.youtube.com
How to Write the Net Ionic Equation for HCl + Ca(OH)2 = CaCl2 + H2O Reaction Between Zinc Oxide And Hydrochloric Acid Formula Balanced equation for the reaction between zinc oxide and dilute hydrochloric acid: The reaction is given below. During this reaction, the zinc metal reacts with the hydrochloric acid to produce zinc chloride and hydrogen gas. Zinc oxide is represented by ${\text{zno}}$ and hydrochloric acid by ${\text{hcl}}$. 5.2 oxidation of zinc by hydrochloric acid subjects: Oxidation/reduction, gas forming reaction, acid properties,. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.youtube.com
Ag + HCl (Silver + Hydrochloric acid) Equation YouTube Reaction Between Zinc Oxide And Hydrochloric Acid Formula In the process, hydrogen gas is produced. Metal oxides are generally basic in nature, therefore, zinc oxide will. This reaction is an example of a single displacement reaction, where one element replaces. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From loecqvsbo.blob.core.windows.net
Copper Hydroxide + Hydrochloric Acid at Joseph Keeble blog Reaction Between Zinc Oxide And Hydrochloric Acid Formula Metal oxides are generally basic in nature, therefore, zinc oxide will. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. The reaction is given below. This reaction is an example of a single displacement reaction, where one element replaces. In the process, hydrogen gas is produced. Zinc is oxidized by hydrochloric acid to form zinc chloride. The reaction. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.youtube.com
Reaction of Zinc with dilute hydrochloric acid or sulphuric acid....Zn Reaction Between Zinc Oxide And Hydrochloric Acid Formula During this reaction, the zinc metal reacts with the hydrochloric acid to produce zinc chloride and hydrogen gas. Zinc oxide is represented by ${\text{zno}}$ and hydrochloric acid by ${\text{hcl}}$. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. Balanced equation for the reaction between zinc oxide and dilute hydrochloric acid: In the process, hydrogen gas is produced. The. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From mungfali.com
PPT Section 9.1 Naming Ions PowerPoint Presentation Reaction Between Zinc Oxide And Hydrochloric Acid Formula Zinc is oxidized by hydrochloric acid to form zinc chloride. Balanced equation for the reaction between zinc oxide and dilute hydrochloric acid: During this reaction, the zinc metal reacts with the hydrochloric acid to produce zinc chloride and hydrogen gas. This reaction is an example of a single displacement reaction, where one element replaces. The reaction between zinc oxide (zno). Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From studylib.net
Rate of reactions chemicalminds Reaction Between Zinc Oxide And Hydrochloric Acid Formula The reaction is given below. Balanced equation for the reaction between zinc oxide and dilute hydrochloric acid: The reaction between zinc oxide (zno) and dilute. Zinc oxide is represented by ${\text{zno}}$ and hydrochloric acid by ${\text{hcl}}$. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. This reaction is an example of a single displacement reaction, where one element. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From hedycabrera.blogspot.com
View Calcium Hydroxide And Hydrochloric Acid Reaction US Reaction Between Zinc Oxide And Hydrochloric Acid Formula This reaction is an example of a single displacement reaction, where one element replaces. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. 5.2 oxidation of zinc by hydrochloric acid subjects: In the process, hydrogen gas is produced. During this reaction, the zinc metal reacts with the hydrochloric acid to produce zinc chloride and hydrogen gas. The reaction. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.numerade.com
SOLVED Consider the reaction between zinc metal and hydrochloric acid Reaction Between Zinc Oxide And Hydrochloric Acid Formula Zinc is oxidized by hydrochloric acid to form zinc chloride. Zinc oxide is represented by ${\text{zno}}$ and hydrochloric acid by ${\text{hcl}}$. Metal oxides are generally basic in nature, therefore, zinc oxide will. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. 5.2. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Reaction Between Zinc Oxide And Hydrochloric Acid Formula During this reaction, the zinc metal reacts with the hydrochloric acid to produce zinc chloride and hydrogen gas. The reaction between zinc oxide (zno) and dilute. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. This reaction is an example of a. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Reaction Between Zinc Oxide And Hydrochloric Acid Formula In the process, hydrogen gas is produced. The reaction between zinc oxide (zno) and dilute. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. Zinc is oxidized by hydrochloric acid to. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.numerade.com
SOLVED explain the following reaction with the help of balance Reaction Between Zinc Oxide And Hydrochloric Acid Formula In the process, hydrogen gas is produced. Metal oxides are generally basic in nature, therefore, zinc oxide will. The reaction between zinc oxide (zno) and dilute. During this reaction, the zinc metal reacts with the hydrochloric acid to produce zinc chloride and hydrogen gas. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. Zinc is oxidized by hydrochloric. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.answersarena.com
[Solved] 2. Zinc chloride can be prepared in the laborator Reaction Between Zinc Oxide And Hydrochloric Acid Formula Zinc is oxidized by hydrochloric acid to form zinc chloride. The reaction is given below. Zinc oxide is represented by ${\text{zno}}$ and hydrochloric acid by ${\text{hcl}}$. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. The reaction between zinc oxide (zno) and dilute. This reaction is an example of a single displacement reaction, where one element replaces. 5.2. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From asideload7.gitlab.io
Ideal Balanced Chemical Equation Hcl And Naoh Equations Worksheet With Reaction Between Zinc Oxide And Hydrochloric Acid Formula 5.2 oxidation of zinc by hydrochloric acid subjects: Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. The reaction between zinc oxide (zno) and dilute. Metal oxides are generally basic in nature, therefore, zinc oxide will. The reaction is given below. During. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.nagwa.com
Question Video Identifying the Salt Produced in an AcidBase Reaction Reaction Between Zinc Oxide And Hydrochloric Acid Formula Zinc is oxidized by hydrochloric acid to form zinc chloride. The reaction is given below. This reaction is an example of a single displacement reaction, where one element replaces. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn]. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From express.adobe.com
Zinc and Hydrochloric Acid Reaction Between Zinc Oxide And Hydrochloric Acid Formula This reaction is an example of a single displacement reaction, where one element replaces. Balanced equation for the reaction between zinc oxide and dilute hydrochloric acid: Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. The reaction between zinc oxide (zno) and. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.slideserve.com
PPT Metal Reactions and Reactivity PowerPoint Presentation, free Reaction Between Zinc Oxide And Hydrochloric Acid Formula The reaction is given below. Balanced equation for the reaction between zinc oxide and dilute hydrochloric acid: Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. Zinc is oxidized by hydrochloric. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.chegg.com
Solved Choose balanced molecular, ionic, and net ionic Reaction Between Zinc Oxide And Hydrochloric Acid Formula This reaction is an example of a single displacement reaction, where one element replaces. During this reaction, the zinc metal reacts with the hydrochloric acid to produce zinc chloride and hydrogen gas. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. The reaction is given below. The reaction between zinc oxide (zno) and dilute. Zinc oxide is represented. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.hanlin.com
Edexcel IGCSE Chemistry 复习笔记 2.7.3 Reactions of Acids翰林国际教育 Reaction Between Zinc Oxide And Hydrochloric Acid Formula The reaction between zinc oxide (zno) and dilute. Zinc is oxidized by hydrochloric acid to form zinc chloride. In the process, hydrogen gas is produced. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. Zinc oxide is represented by ${\text{zno}}$ and hydrochloric. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.youtube.com
How to Balance Zn + NaOH = Na2ZnO2 + H2 (Zinc + Sodium hydroxide) YouTube Reaction Between Zinc Oxide And Hydrochloric Acid Formula Zinc is oxidized by hydrochloric acid to form zinc chloride. The reaction is given below. 5.2 oxidation of zinc by hydrochloric acid subjects: Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. In the process, hydrogen gas is produced. Metal oxides are. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.linstitute.net
CIE A Level Chemistry复习笔记2.2.2 Reactions of Group 2 Oxides, Hydroxides Reaction Between Zinc Oxide And Hydrochloric Acid Formula Metal oxides are generally basic in nature, therefore, zinc oxide will. Balanced equation for the reaction between zinc oxide and dilute hydrochloric acid: Zinc oxide is represented by ${\text{zno}}$ and hydrochloric acid by ${\text{hcl}}$. 5.2 oxidation of zinc by hydrochloric acid subjects: In the process, hydrogen gas is produced. During this reaction, the zinc metal reacts with the hydrochloric acid. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.youtube.com
Zinc and 6 M Hydrochloric Acid Reaction YouTube Reaction Between Zinc Oxide And Hydrochloric Acid Formula 5.2 oxidation of zinc by hydrochloric acid subjects: The reaction is given below. This reaction is an example of a single displacement reaction, where one element replaces. The reaction between zinc oxide (zno) and dilute. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. During this reaction, the zinc metal reacts with the hydrochloric acid to produce zinc. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Reaction Between Zinc Oxide And Hydrochloric Acid Formula In the process, hydrogen gas is produced. The reaction between zinc oxide (zno) and dilute. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. The reaction is given below. Zinc is oxidized by hydrochloric acid to form zinc chloride. 5.2 oxidation of zinc by hydrochloric acid subjects: Balanced equation for the reaction between zinc oxide and dilute hydrochloric. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From brainly.in
What are amphoteric oxides. Explain with the help of chemical reactions Reaction Between Zinc Oxide And Hydrochloric Acid Formula In the process, hydrogen gas is produced. This reaction is an example of a single displacement reaction, where one element replaces. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen chloride. The reaction is given below. During this reaction, the zinc metal reacts. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.
From www.youtube.com
Reaction Of Zinc with Hydrochloric acid Chemistry demonstration YouTube Reaction Between Zinc Oxide And Hydrochloric Acid Formula Zinc oxide is represented by ${\text{zno}}$ and hydrochloric acid by ${\text{hcl}}$. In the process, hydrogen gas is produced. The reaction is given below. Balanced equation for the reaction between zinc oxide and dilute hydrochloric acid: 5.2 oxidation of zinc by hydrochloric acid subjects: During this reaction, the zinc metal reacts with the hydrochloric acid to produce zinc chloride and hydrogen. Reaction Between Zinc Oxide And Hydrochloric Acid Formula.