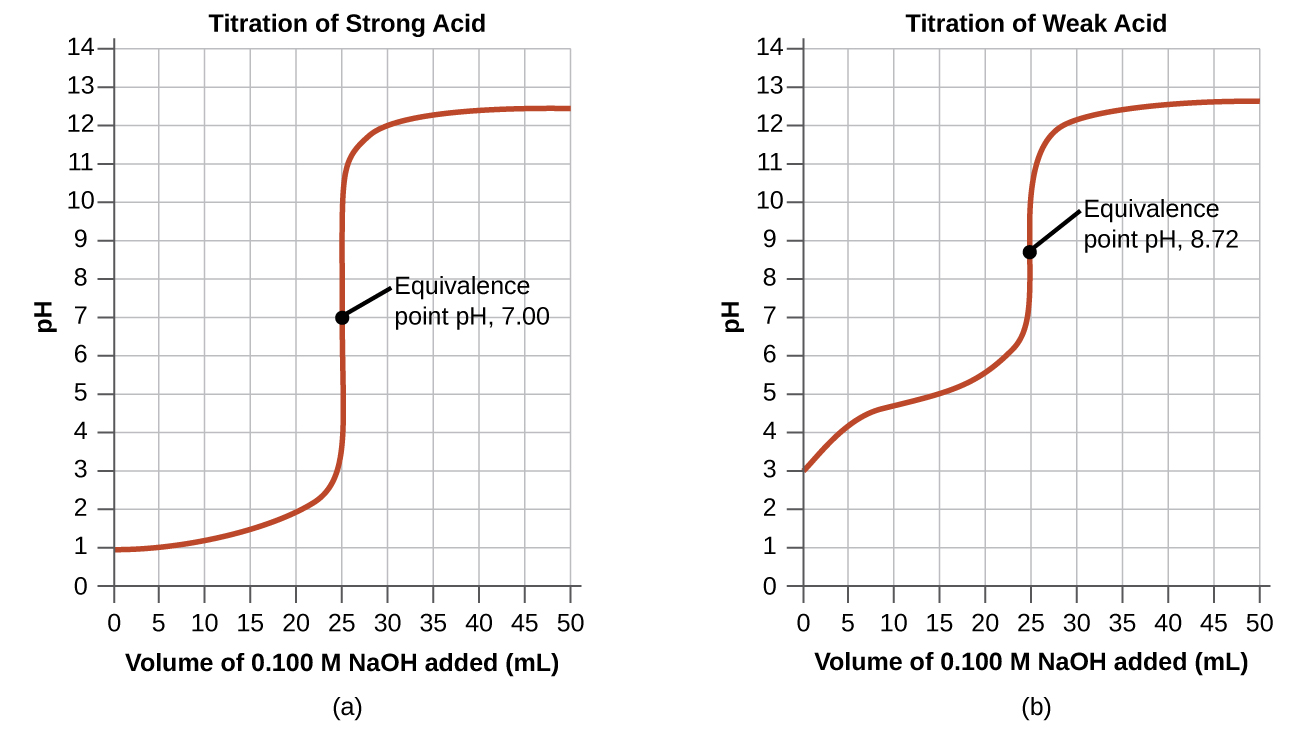
Titration Equivalence Point Concentrations . Suppose that a titration is performed and \(20.70 \: Equivalence point calculations are discussed in more details in the appropriate sections: As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. \ce{naoh}\) is required to reach the end point when titrated. Sorting out some confusing terms. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). In this section, we will explore the. An indicator that changes colour at a. If either the titrant or. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are 0.1 m. Equivalence points are not always ph 7. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. The equivalence point of a titration.
from psu.pb.unizin.org
The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). Suppose that a titration is performed and \(20.70 \: As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. Equivalence point calculations are discussed in more details in the appropriate sections: The equivalence point of a titration. An indicator that changes colour at a. Equivalence points are not always ph 7. If either the titrant or. \ce{naoh}\) is required to reach the end point when titrated. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are 0.1 m.
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax
Titration Equivalence Point Concentrations If either the titrant or. In this section, we will explore the. Sorting out some confusing terms. Suppose that a titration is performed and \(20.70 \: The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. An indicator that changes colour at a. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are 0.1 m. Equivalence point calculations are discussed in more details in the appropriate sections: The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. If either the titrant or. Equivalence points are not always ph 7. \ce{naoh}\) is required to reach the end point when titrated. The equivalence point of a titration.
From www.chegg.com
Solved When titrating nitrous acid with barium hydroxide at Titration Equivalence Point Concentrations The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are 0.1 m. Suppose that a titration is performed and \(20.70 \: In this section, we will explore the. The. Titration Equivalence Point Concentrations.
From exyjcpegf.blob.core.windows.net
Titration And Equivalence Point at Bill Hubbard blog Titration Equivalence Point Concentrations In this section, we will explore the. Equivalence points are not always ph 7. Sorting out some confusing terms. An indicator that changes colour at a. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. \ce{naoh}\) is required to reach the end point when titrated. Suppose that a. Titration Equivalence Point Concentrations.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration Equivalence Point Concentrations In this section, we will explore the. An indicator that changes colour at a. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Sorting out some confusing terms. Equivalence point calculations are discussed in more details in the appropriate sections: If either the titrant or. The equivalence point. Titration Equivalence Point Concentrations.
From joizlgxlm.blob.core.windows.net
Titration Experiments In Supramolecular Chemistry at Larry Ferrill blog Titration Equivalence Point Concentrations An indicator that changes colour at a. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are 0.1 m.. Titration Equivalence Point Concentrations.
From www.youtube.com
Lecture 7 Titration Half Equivalence Point YouTube Titration Equivalence Point Concentrations The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). If either the titrant or. Calculate ph at. Titration Equivalence Point Concentrations.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Titration Equivalence Point Concentrations As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. If either the titrant or. The equivalence point is the point at which titrant. Titration Equivalence Point Concentrations.
From schoolbag.info
What volume of NaOH( aq ) would be needed to reach the equivalence Titration Equivalence Point Concentrations The equivalence point of a titration. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are 0.1 m. \ce{naoh}\) is required to reach the end point when titrated. Equivalence points are not always ph 7. Sorting out some confusing terms. The equivalence point of a titration is the point at. Titration Equivalence Point Concentrations.
From www.chegg.com
Solved The titration of a strong acid with a weak base will Titration Equivalence Point Concentrations An indicator that changes colour at a. If either the titrant or. Sorting out some confusing terms. The equivalence point of a titration. Suppose that a titration is performed and \(20.70 \: Equivalence points are not always ph 7. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed.. Titration Equivalence Point Concentrations.
From uhighlsu.web.fc2.com
Help Titration Equivalence Point Concentrations Sorting out some confusing terms. \ce{naoh}\) is required to reach the end point when titrated. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. An indicator that changes colour at a. In this section, we will explore the. The equivalence point of a titration. Titration Equivalence Point Concentrations.
From www.tes.com
Acid Base Titration Theory, Titration Lab, Titration Stoichiometry Titration Equivalence Point Concentrations The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). Suppose that a titration is performed and \(20.70 \: Sorting out some confusing terms. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant. Titration Equivalence Point Concentrations.
From www.chegg.com
Solved QUESTION 12What is the pH at the point in a titration Titration Equivalence Point Concentrations Equivalence point calculations are discussed in more details in the appropriate sections: Equivalence points are not always ph 7. Suppose that a titration is performed and \(20.70 \: Sorting out some confusing terms. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. If either the titrant or. In. Titration Equivalence Point Concentrations.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Titration Equivalence Point Concentrations The equivalence point of a titration. Equivalence points are not always ph 7. If either the titrant or. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. In this section, we will explore the. Sorting out some confusing terms. Equivalence point calculations are discussed. Titration Equivalence Point Concentrations.
From exovodadv.blob.core.windows.net
Titration End Point Meaning at Jenny Ingram blog Titration Equivalence Point Concentrations An indicator that changes colour at a. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Equivalence points are not always ph 7. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles. Titration Equivalence Point Concentrations.
From exoaqbcwf.blob.core.windows.net
Equivalence Point Titration With Base at Alvin Barajas blog Titration Equivalence Point Concentrations Equivalence point calculations are discussed in more details in the appropriate sections: The equivalence point of a titration. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are 0.1 m. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. As. Titration Equivalence Point Concentrations.
From forums.studentdoctor.net
Confusion about titration of polyprotic acids Student Doctor Network Titration Equivalence Point Concentrations Sorting out some confusing terms. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are 0.1 m. The equivalence point of a titration. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. In this section, we will explore. Titration Equivalence Point Concentrations.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Equivalence Point Concentrations Suppose that a titration is performed and \(20.70 \: The equivalence point of a titration. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. \ce{naoh}\) is required to reach the end point when titrated. Sorting out some confusing terms. Calculate ph at the equivalence. Titration Equivalence Point Concentrations.
From saylordotorg.github.io
AcidBase Titrations Titration Equivalence Point Concentrations Suppose that a titration is performed and \(20.70 \: The equivalence point of a titration. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are 0.1 m. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Titration Equivalence Point Concentrations.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Equivalence Point Concentrations In this section, we will explore the. An indicator that changes colour at a. Sorting out some confusing terms. Equivalence point calculations are discussed in more details in the appropriate sections: The equivalence point of a titration. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are 0.1 m. Equivalence. Titration Equivalence Point Concentrations.
From www.reddit.com
Is (half equivalence point) when a conjugate base and an acid are equal Titration Equivalence Point Concentrations The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. In this section, we will explore the. Equivalence point calculations are discussed in more details in the appropriate sections: The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically. Titration Equivalence Point Concentrations.
From chemistrytalk.org
Titration Curves & Equivalence Point Calculations ChemTalk Titration Equivalence Point Concentrations \ce{naoh}\) is required to reach the end point when titrated. If either the titrant or. Suppose that a titration is performed and \(20.70 \: The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). Equivalence points are not always. Titration Equivalence Point Concentrations.
From chemistry.stackexchange.com
Why does the pH before the equivalence point of a titration depend on Titration Equivalence Point Concentrations In this section, we will explore the. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. The equivalence point of a titration. An indicator that changes colour at a. Sorting out some confusing terms. \ce{naoh}\) is required to reach the end point when titrated.. Titration Equivalence Point Concentrations.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Equivalence Point Concentrations The equivalence point of a titration. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are 0.1 m. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). \ce{naoh}\). Titration Equivalence Point Concentrations.
From webmis.highland.cc.il.us
AcidBase Titrations Titration Equivalence Point Concentrations In this section, we will explore the. Sorting out some confusing terms. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Suppose that a titration is performed and \(20.70 \: An indicator that changes colour at a. \ce{naoh}\) is required to reach the end point when titrated. Calculate. Titration Equivalence Point Concentrations.
From joimexzcj.blob.core.windows.net
Ph Titration Definition at Michele Esposito blog Titration Equivalence Point Concentrations Suppose that a titration is performed and \(20.70 \: As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. The equivalence point of a titration. Equivalence points are not always ph 7. Equivalence point calculations are discussed in more details in the appropriate sections: Calculate. Titration Equivalence Point Concentrations.
From www.numerade.com
SOLVEDThe graph shows the titration curves for two Titration Equivalence Point Concentrations Equivalence point calculations are discussed in more details in the appropriate sections: Equivalence points are not always ph 7. Sorting out some confusing terms. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are 0.1 m. Suppose that a titration is performed and \(20.70 \: The equivalence point is the. Titration Equivalence Point Concentrations.
From chem.libretexts.org
11.3 Reaction Stoichiometry in Solutions AcidBase Titrations Titration Equivalence Point Concentrations Equivalence points are not always ph 7. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid. Titration Equivalence Point Concentrations.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration Equivalence Point Concentrations \ce{naoh}\) is required to reach the end point when titrated. Equivalence point calculations are discussed in more details in the appropriate sections: The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). An indicator that changes colour at a.. Titration Equivalence Point Concentrations.
From joiyrusdv.blob.core.windows.net
How To Find Titration Equivalence Point at Douglas Fuller blog Titration Equivalence Point Concentrations If either the titrant or. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Equivalence points are. Titration Equivalence Point Concentrations.
From joiyrusdv.blob.core.windows.net
How To Find Titration Equivalence Point at Douglas Fuller blog Titration Equivalence Point Concentrations \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \: The equivalence point of a titration. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). The equivalence point of. Titration Equivalence Point Concentrations.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Equivalence Point Concentrations The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both. Titration Equivalence Point Concentrations.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Equivalence Point Concentrations Equivalence point calculations are discussed in more details in the appropriate sections: An indicator that changes colour at a. \ce{naoh}\) is required to reach the end point when titrated. In this section, we will explore the. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are 0.1 m. The equivalence. Titration Equivalence Point Concentrations.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration Equivalence Point Concentrations An indicator that changes colour at a. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. In this section, we will explore the. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are 0.1. Titration Equivalence Point Concentrations.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Equivalence Point Concentrations \ce{naoh}\) is required to reach the end point when titrated. Equivalence points are not always ph 7. Sorting out some confusing terms. An indicator that changes colour at a. Suppose that a titration is performed and \(20.70 \: The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. If. Titration Equivalence Point Concentrations.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder Titration Equivalence Point Concentrations Suppose that a titration is performed and \(20.70 \: The equivalence point of a titration. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. \ce{naoh}\) is required to reach the end point when titrated. In this section, we will explore the. If either the. Titration Equivalence Point Concentrations.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point Titration Equivalence Point Concentrations Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are 0.1 m. Sorting out some confusing terms. An indicator that changes colour at a. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Equivalence point calculations are discussed. Titration Equivalence Point Concentrations.