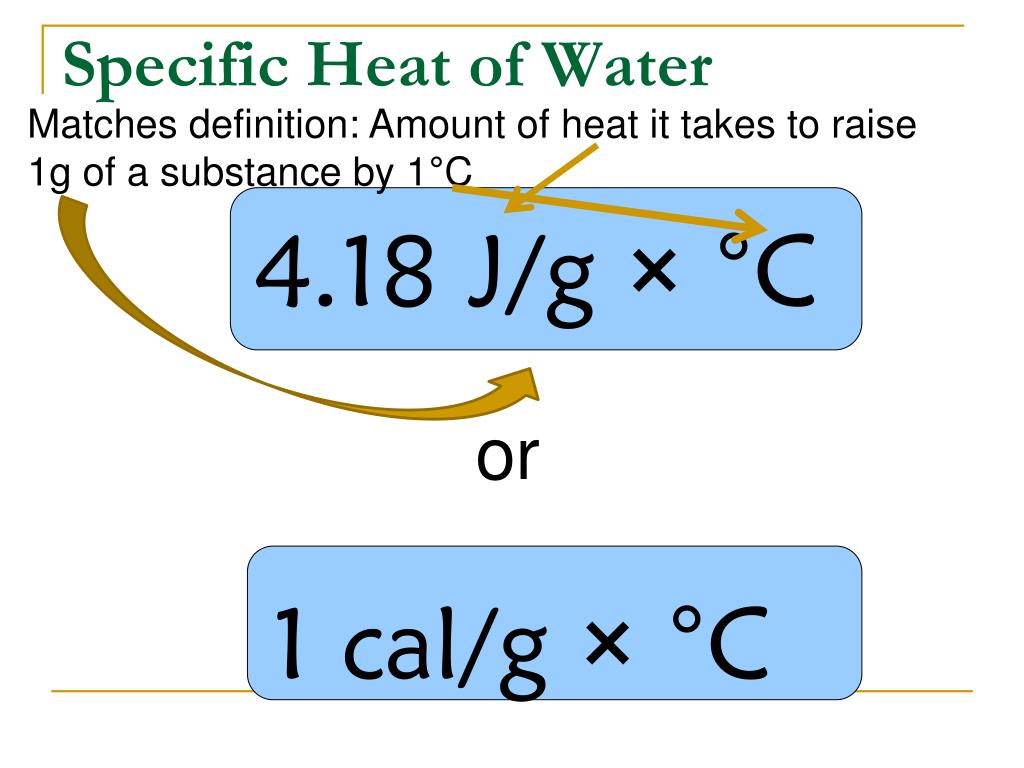
Define Heating Of Water . Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat is recorded in calories for “ mass in grams ” (and “joules for kg”). Water has a particularly high specific heat compared to many other substances. The stove, pot, water, and steam all have different specific heats. In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific heat is so high. The heart of a hot water heating system is usually a boiler. In a recirculating system, which is most common these days, hot water actively circulates through the. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Specific heat is a measure of the energy required to heat 1 gram of substance 1° c. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. Thermal energy, in turn, is the kinetic energy of. Heat is the thermal energy transfer between systems or bodies due to a temperature difference.
from www.slideserve.com
Specific heat is a measure of the energy required to heat 1 gram of substance 1° c. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). In a recirculating system, which is most common these days, hot water actively circulates through the. The heart of a hot water heating system is usually a boiler. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat is recorded in calories for “ mass in grams ” (and “joules for kg”). Thermal energy, in turn, is the kinetic energy of. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. Water has a particularly high specific heat compared to many other substances. In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific heat is so high.
PPT Unit 09 PowerPoint Presentation, free download ID5621270
Define Heating Of Water Specific heat is a measure of the energy required to heat 1 gram of substance 1° c. In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific heat is so high. The heart of a hot water heating system is usually a boiler. Specific heat is a measure of the energy required to heat 1 gram of substance 1° c. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat is recorded in calories for “ mass in grams ” (and “joules for kg”). Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Heat is the thermal energy transfer between systems or bodies due to a temperature difference. In a recirculating system, which is most common these days, hot water actively circulates through the. Thermal energy, in turn, is the kinetic energy of. The stove, pot, water, and steam all have different specific heats. Water has a particularly high specific heat compared to many other substances.
From colosoimages.com
convection currents labeled diagram Coloso Define Heating Of Water The heart of a hot water heating system is usually a boiler. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Our water heating calculator can help you determine both the. Define Heating Of Water.
From ch301.cm.utexas.edu
heating curve Define Heating Of Water The heart of a hot water heating system is usually a boiler. Specific heat is recorded in calories for “ mass in grams ” (and “joules for kg”). Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Our water heating calculator can help you determine both the amount of heat required to raise the temperature. Define Heating Of Water.
From me-mechanicalengineering.com
Modes of Heat Transfer Define Heating Of Water Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat is recorded in calories for “ mass in grams ” (and “joules for kg”). Specific. Define Heating Of Water.
From www.slideserve.com
PPT Molecules of Life PowerPoint Presentation, free download ID1321531 Define Heating Of Water Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat is recorded in calories for “ mass in grams ” (and “joules for kg”). Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. In. Define Heating Of Water.
From www.dreamstime.com
Thermal Energy Physics Definition, Example with Water and Define Heating Of Water In a recirculating system, which is most common these days, hot water actively circulates through the. The stove, pot, water, and steam all have different specific heats. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Water has a particularly high specific heat compared to. Define Heating Of Water.
From www.nuclear-power.com
Convection Convective Heat Transfer Definition Define Heating Of Water The heart of a hot water heating system is usually a boiler. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat is a measure of the energy required to heat 1 gram of substance 1° c. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Thermal energy,. Define Heating Of Water.
From studylib.net
1.3 Specific Heat Capacity Define Heating Of Water Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. Specific heat is recorded in calories for “ mass in grams ” (and “joules for kg”). Heat is the thermal energy transfer between systems or bodies due to a temperature difference.. Define Heating Of Water.
From www.richhoffmanclass.com
PHY 116 Chapter 2 Define Heating Of Water Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Specific heat is a measure of the. Define Heating Of Water.
From chem.libretexts.org
11.7 Heating Curve for Water Chemistry LibreTexts Define Heating Of Water In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific heat is so high. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Thermal energy, in turn, is the kinetic energy of. Specific heat is defined by the amount of heat needed to. Define Heating Of Water.
From cuagodep.net
What Is The Molar Heat Capacity Of Water Unraveling Its Thermal Mysteries Define Heating Of Water In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific heat is so high. The heart of a hot water heating system is usually a boiler. Specific heat is recorded in calories for “ mass in grams ” (and “joules for kg”). Specific heat is defined by the. Define Heating Of Water.
From www.showme.com
Properties of water high heat capacity Science ShowMe Define Heating Of Water Specific heat is a measure of the energy required to heat 1 gram of substance 1° c. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). In a recirculating system, which is most common these days, hot water actively circulates through the. Specific heat is. Define Heating Of Water.
From www.cashify.in
What Is Solar Water Heating System And How Does it Work? Cashify Blog Define Heating Of Water Water has a particularly high specific heat compared to many other substances. In a recirculating system, which is most common these days, hot water actively circulates through the. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. The heart of. Define Heating Of Water.
From lewismingibbs.blogspot.com
Convectional Heating Is Best Described as LewisminGibbs Define Heating Of Water Specific heat is a measure of the energy required to heat 1 gram of substance 1° c. Thermal energy, in turn, is the kinetic energy of. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Water has a particularly high specific heat compared to many. Define Heating Of Water.
From worksheetfullfunniest.z21.web.core.windows.net
Heat Energy During Phase Change Define Heating Of Water Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat is recorded in calories for “ mass in grams ” (and “joules for kg”). In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific heat is so high. Specific heat is a. Define Heating Of Water.
From www.studyiq.com
Heat Transfer Types, Definition, Convection, Radiation, Conduction Define Heating Of Water The heart of a hot water heating system is usually a boiler. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Water has a particularly high specific heat compared to many other substances. In this article, we’ll be covering what specific heat is, what equation. Define Heating Of Water.
From www.tessshebaylo.com
Water Heating Equation Tessshebaylo Define Heating Of Water In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific heat is so high. The stove, pot, water, and steam all have different specific heats. Specific heat is a measure of the energy required to heat 1 gram of substance 1° c. Specific heat is defined by the. Define Heating Of Water.
From www.chemicalslearning.com
Types of Convection Heat Transfer, Examples and Rate of Heat Transfer Define Heating Of Water Specific heat is a measure of the energy required to heat 1 gram of substance 1° c. In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific heat is so high. In a recirculating system, which is most common these days, hot water actively circulates through the. Heat. Define Heating Of Water.
From www.sciencefacts.net
Heat Transfer Definition, Types, And Examples Define Heating Of Water The heart of a hot water heating system is usually a boiler. In a recirculating system, which is most common these days, hot water actively circulates through the. Specific heat is recorded in calories for “ mass in grams ” (and “joules for kg”). Our water heating calculator can help you determine both the amount of heat required to raise. Define Heating Of Water.
From arenahanna.wordpress.com
HEAT WORLD OF PHYSICS steps by steps to understand heat Define Heating Of Water Specific heat is recorded in calories for “ mass in grams ” (and “joules for kg”). Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. Specific heat is a measure of the energy required to heat 1 gram of substance. Define Heating Of Water.
From www.vectorstock.com
Diagram showing how heat transfer Royalty Free Vector Image Define Heating Of Water The stove, pot, water, and steam all have different specific heats. Specific heat is a measure of the energy required to heat 1 gram of substance 1° c. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. In a recirculating. Define Heating Of Water.
From www.slideserve.com
PPT Unit 09 PowerPoint Presentation, free download ID5621270 Define Heating Of Water Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Water has a particularly high specific heat compared to many other substances. In a recirculating system, which is most common these days, hot water actively circulates through the. Thermal energy, in turn, is the kinetic energy of. The heart of a hot water heating system is. Define Heating Of Water.
From www.slideserve.com
PPT AP Biology Ch. 3 PowerPoint Presentation, free download ID1989378 Define Heating Of Water Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Water has a particularly high specific heat compared to many other substances. The heart of a hot water heating system is usually. Define Heating Of Water.
From www.slideserve.com
PPT Energy and Temperature PowerPoint Presentation, free download Define Heating Of Water Specific heat is a measure of the energy required to heat 1 gram of substance 1° c. Water has a particularly high specific heat compared to many other substances. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram. Define Heating Of Water.
From missehonorsbio.blogspot.com
EC Honors Biology Specific Heat and Water as a Solvent Define Heating Of Water In a recirculating system, which is most common these days, hot water actively circulates through the. The stove, pot, water, and steam all have different specific heats. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Thermal energy, in turn, is the kinetic energy of. Water has a particularly high specific heat compared to many. Define Heating Of Water.
From lessondbmetalepses.z21.web.core.windows.net
Conduction Convection Radiation Lab Activity Define Heating Of Water Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. The heart of a hot water heating system is usually a boiler. Thermal energy, in turn, is the kinetic energy of. Specific heat is recorded in calories for “ mass in. Define Heating Of Water.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Define Heating Of Water Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Specific heat is recorded in calories for “ mass in grams ” (and “joules for kg”). Water has a particularly high specific heat compared to many other substances. Our water heating calculator can help you determine. Define Heating Of Water.
From apollo.lsc.vsc.edu
Latent Heats sublimation and deposition Define Heating Of Water Heat is the thermal energy transfer between systems or bodies due to a temperature difference. In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific heat is so high. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of. Define Heating Of Water.
From heatklp.blogspot.com
11+ Why Is Heat Of Vaporization Important To Life On Earth Article Define Heating Of Water Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. Water has a particularly high specific heat compared to many other substances. Thermal energy, in turn, is the kinetic energy of. The stove, pot, water, and steam all have different specific. Define Heating Of Water.
From becuo.com
Heat Transfer Images & Pictures Becuo Define Heating Of Water Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Water has a particularly high specific heat compared to many other substances. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and. Define Heating Of Water.
From mavink.com
Convection Currents Model Define Heating Of Water In a recirculating system, which is most common these days, hot water actively circulates through the. Water has a particularly high specific heat compared to many other substances. Specific heat is recorded in calories for “ mass in grams ” (and “joules for kg”). In this article, we’ll be covering what specific heat is, what equation you use to find. Define Heating Of Water.
From www.britannica.com
Convection Definition, Examples, Types, & Facts Britannica Define Heating Of Water Specific heat is recorded in calories for “ mass in grams ” (and “joules for kg”). Specific heat is a measure of the energy required to heat 1 gram of substance 1° c. Thermal energy, in turn, is the kinetic energy of. Our water heating calculator can help you determine both the amount of heat required to raise the temperature. Define Heating Of Water.
From www.geeksforgeeks.org
Specific Heat Capacity Definition, Formula, Water Heat Capacity Define Heating Of Water In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific heat is so high. In a recirculating system, which is most common these days, hot water actively circulates through the. Water has a particularly high specific heat compared to many other substances. Specific heat is defined by the. Define Heating Of Water.
From www.slideserve.com
PPT High Specific Heat of Water PowerPoint Presentation, free Define Heating Of Water The heart of a hot water heating system is usually a boiler. The stove, pot, water, and steam all have different specific heats. Water has a particularly high specific heat compared to many other substances. In a recirculating system, which is most common these days, hot water actively circulates through the. Specific heat is recorded in calories for “ mass. Define Heating Of Water.
From whatsinsight.org
What is the Specific Heat of Water? What's Insight Define Heating Of Water Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Heat is the thermal energy transfer between systems or bodies due to a temperature difference. The heart of a hot water heating system is usually a boiler. Specific heat is a measure of the energy required. Define Heating Of Water.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Define Heating Of Water Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Specific heat is recorded in calories for “ mass in grams ” (and “joules for kg”). The heart of a hot water heating system is usually a boiler. Thermal energy, in turn, is the kinetic energy of. In this article, we’ll be covering what specific. Define Heating Of Water.