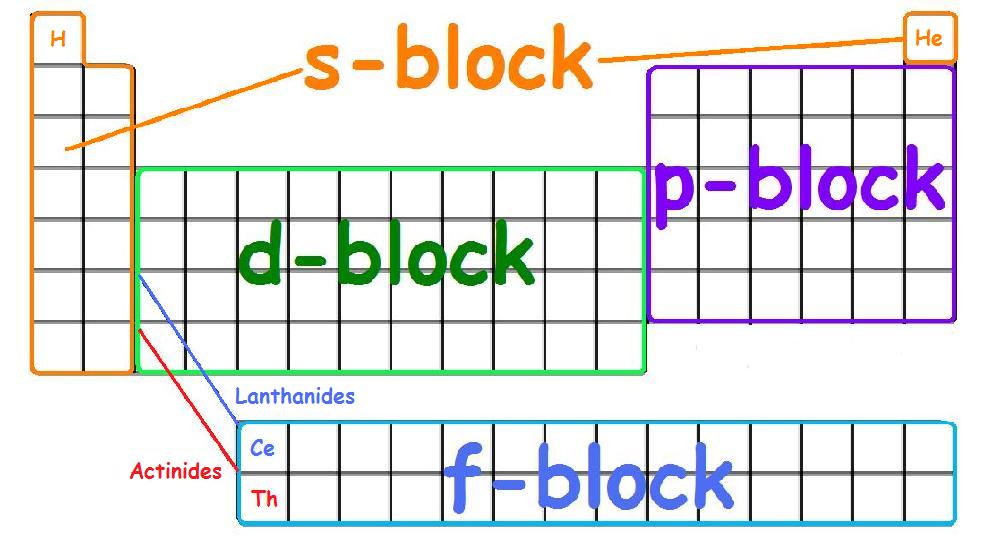
Full Form Of S P D F Subshell . Each subshell can hold a different number of electrons. A subshell is a subdivision of electron shells separated by electron orbitals. There are 4 subshells, s, p, d, and f. How would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? Within each shell of an atom there are some combinations of orbitals. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). Subshells are labelled s, p, d, and f in an electron configuration. How many d orbitals must be occupied by single. ⚛ group 1 elements occur at the. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. The n number determines how many of the subshells make up the shell. How many 2p orbitals are there in an atom? The labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. The s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up to 10 electrons, and the f. The maximum number of electrons that can be accommodated by a subshell.
from chemwiki.ucdavis.edu
⚛ group 1 elements occur at the. The labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. How many 2p orbitals are there in an atom? There are 4 subshells, s, p, d, and f. Subshells are labelled s, p, d, and f in an electron configuration. The n number determines how many of the subshells make up the shell. The maximum number of electrons that can be accommodated by a subshell. Each subshell can hold a different number of electrons. Within each shell of an atom there are some combinations of orbitals. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental).
Electron Configuration of Transition Metals Chemwiki
Full Form Of S P D F Subshell The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. ⚛ group 1 elements occur at the. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). The s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up to 10 electrons, and the f. There are 4 subshells, s, p, d, and f. How would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. The maximum number of electrons that can be accommodated by a subshell. Within each shell of an atom there are some combinations of orbitals. Each subshell can hold a different number of electrons. How many d orbitals must be occupied by single. How many 2p orbitals are there in an atom? A subshell is a subdivision of electron shells separated by electron orbitals. Subshells are labelled s, p, d, and f in an electron configuration. The n number determines how many of the subshells make up the shell. The labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons.
From gbu-taganskij.ru
SPDF Orbitals Explained Quantum Numbers, Electron, 53 OFF Full Form Of S P D F Subshell There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). Within each shell of an atom there are some combinations of orbitals. ⚛ group 1 elements occur at the. A subshell is a subdivision of electron shells separated by electron orbitals. How many 2p orbitals are there in. Full Form Of S P D F Subshell.
From byjusexamprep.com
What is the Full Form of SST Subject? SST Full Form Full Form Of S P D F Subshell The n number determines how many of the subshells make up the shell. Each subshell can hold a different number of electrons. A subshell is a subdivision of electron shells separated by electron orbitals. The labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. There are 4 subshells,. Full Form Of S P D F Subshell.
From www.quora.com
What is the full form of S, P, D, and F? Quora Full Form Of S P D F Subshell Within each shell of an atom there are some combinations of orbitals. The labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). There are 4 subshells,. Full Form Of S P D F Subshell.
From www.youtube.com
Full form of s,p,d,f YouTube Full Form Of S P D F Subshell The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). There are 4 subshells, s, p, d, and f. The labels s, p, d and f blocks of. Full Form Of S P D F Subshell.
From www.scribd.com
All Full Forms for Computer Subject.pdf Domain Name World Wide Full Form Of S P D F Subshell The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. The s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up to 10 electrons, and the. Full Form Of S P D F Subshell.
From worksheethelsugw5.z21.web.core.windows.net
Chem Reference Sheet For Nomenclature Full Form Of S P D F Subshell The maximum number of electrons that can be accommodated by a subshell. ⚛ group 1 elements occur at the. How would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? Subshells are labelled s, p, d, and f in an electron configuration. The n number determines how many of the subshells make up the shell.. Full Form Of S P D F Subshell.
From www.youtube.com
S.A का full form क्या है ? Full form of sa in english YouTube Full Form Of S P D F Subshell The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. The maximum number of electrons that can be accommodated by a subshell. Subshells are labelled s, p, d, and f in an electron configuration. ⚛ group 1 elements occur at the. How many d orbitals must be occupied by single.. Full Form Of S P D F Subshell.
From www.scribd.com
What Is The Full Form of S.T.O.R.E.? PDF Full Form Of S P D F Subshell The maximum number of electrons that can be accommodated by a subshell. Each subshell can hold a different number of electrons. How would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). Within. Full Form Of S P D F Subshell.
From www.youtube.com
How To Find Subshell in Orbit or Shell Full Form of S p d f Full Form Of S P D F Subshell How would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? Each subshell can hold a different number of electrons. A subshell is a subdivision of electron shells separated by electron orbitals. The labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. The. Full Form Of S P D F Subshell.
From www.youtube.com
Full Form of S&P S&P full form S&P means S&P Stands for S&P फुल Full Form Of S P D F Subshell The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. A subshell is a subdivision of electron shells separated by electron orbitals. The maximum number of electrons that can be accommodated by a subshell. There are 4 subshells, s, p, d, and f. Each subshell can hold a different number. Full Form Of S P D F Subshell.
From www.vrogue.co
A Chart Of The Spdf Electron Orbitals Chemistry Educa vrogue.co Full Form Of S P D F Subshell The maximum number of electrons that can be accommodated by a subshell. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. Subshells are labelled s, p, d, and f in an electron configuration. There are 4 subshells, s, p, d, and f. The labels s, p, d and f. Full Form Of S P D F Subshell.
From periodictable.me
Phosphorus Electron Configuration (P) with Orbital Diagram Full Form Of S P D F Subshell The s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up to 10 electrons, and the f. ⚛ group 1 elements occur at the. A subshell is a subdivision of electron shells separated by electron orbitals.. Full Form Of S P D F Subshell.
From hromfy.weebly.com
Periodic table f hromfy Full Form Of S P D F Subshell The maximum number of electrons that can be accommodated by a subshell. A subshell is a subdivision of electron shells separated by electron orbitals. Each subshell can hold a different number of electrons. How many d orbitals must be occupied by single. How would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? ⚛ group. Full Form Of S P D F Subshell.
From www.youtube.com
Full form of SP YouTube Full Form Of S P D F Subshell The labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. How would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? The maximum number of electrons that can be accommodated by a subshell. Each subshell can hold a different number of electrons. The. Full Form Of S P D F Subshell.
From chemwiki.ucdavis.edu
Electron Configuration of Transition Metals Chemwiki Full Form Of S P D F Subshell The labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. The maximum number of electrons that can be accommodated by a subshell. How would you describe the shapes. Full Form Of S P D F Subshell.
From www.educba.com
Full Form of BTS Introduction, Rise, Impact, Fandom & Success Full Form Of S P D F Subshell There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). Each subshell can hold a different number of electrons. There are 4 subshells, s, p, d, and f. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively.. Full Form Of S P D F Subshell.
From studylib.net
S P D F Orbitals Full Form Of S P D F Subshell Within each shell of an atom there are some combinations of orbitals. There are 4 subshells, s, p, d, and f. A subshell is a subdivision of electron shells separated by electron orbitals. Each subshell can hold a different number of electrons. Subshells are labelled s, p, d, and f in an electron configuration. How many d orbitals must be. Full Form Of S P D F Subshell.
From www.youtube.com
full form of ms 😎😎 YouTube Full Form Of S P D F Subshell The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. The n number determines how many of the subshells make up the shell. The maximum number of electrons that can be accommodated by a subshell. There are 4 subshells, s, p, d, and f. Within each shell of an atom. Full Form Of S P D F Subshell.
From abhimanyusir.blogspot.com
10th Chart Full Forms, Abbreviation Full Form Of S P D F Subshell The s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up to 10 electrons, and the f. ⚛ group 1 elements occur at the. The subshells correspond to l=0, l=1, l=2, and l=3 and are named. Full Form Of S P D F Subshell.
From www.youtube.com
full form of s.s.t youtube trending shorts shorts YouTube Full Form Of S P D F Subshell The labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. There are 4 subshells, s, p, d, and f. Within each shell of an atom there are some combinations of orbitals. The s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has. Full Form Of S P D F Subshell.
From ar.inspiredpencil.com
S P D F Orbital Full Form Of S P D F Subshell A subshell is a subdivision of electron shells separated by electron orbitals. Within each shell of an atom there are some combinations of orbitals. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. Subshells are labelled s, p, d, and f in an electron configuration. The labels s, p,. Full Form Of S P D F Subshell.
From www.youtube.com
Full Form of S S full form S ka full form S means S Stands for Full Form Of S P D F Subshell How many 2p orbitals are there in an atom? The n number determines how many of the subshells make up the shell. Each subshell can hold a different number of electrons. Within each shell of an atom there are some combinations of orbitals. The s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has. Full Form Of S P D F Subshell.
From www.youtube.com
What Is the Full Form of sblock , pblock , dblock , fblock neet Full Form Of S P D F Subshell The s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up to 10 electrons, and the f. The maximum number of electrons that can be accommodated by a subshell. There are four types of orbitals that. Full Form Of S P D F Subshell.
From www.youtube.com
Full form of S S C, H S C, I A, I Sc, I Com, B A, B Sc, B Com. M A & M Full Form Of S P D F Subshell The s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up to 10 electrons, and the f. The maximum number of electrons that can be accommodated by a subshell. How would you describe the shapes and. Full Form Of S P D F Subshell.
From in.pinterest.com
Pin on Full Form of words & Products Tag line Interesting english Full Form Of S P D F Subshell The maximum number of electrons that can be accommodated by a subshell. There are 4 subshells, s, p, d, and f. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). Subshells are labelled s, p, d, and f in an electron configuration. Each subshell can hold a. Full Form Of S P D F Subshell.
From www.youtube.com
Full form of SO, CO, SI, SP SSP, ACP, ASI ASP DSP DCP, IPS YouTube Full Form Of S P D F Subshell Within each shell of an atom there are some combinations of orbitals. How would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? A subshell is a subdivision of electron shells separated by electron orbitals. There are 4 subshells, s, p, d, and f. The labels s, p, d and f blocks of the periodic. Full Form Of S P D F Subshell.
From www.showme.com
Electron configuration example for phosphorus Science, Chemistry Full Form Of S P D F Subshell Each subshell can hold a different number of electrons. Within each shell of an atom there are some combinations of orbitals. How would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? The n number determines how many of the subshells make up the shell. How many 2p orbitals are there in an atom? The. Full Form Of S P D F Subshell.
From gbu-taganskij.ru
SPDF Orbitals Explained Quantum Numbers, Electron, 53 OFF Full Form Of S P D F Subshell How would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? How many 2p orbitals are there in an atom? The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. The labels s, p, d and f blocks of the periodic table refer to the. Full Form Of S P D F Subshell.
From byjus.com
What do s, p, d, f stand for? Full Form Of S P D F Subshell ⚛ group 1 elements occur at the. A subshell is a subdivision of electron shells separated by electron orbitals. The maximum number of electrons that can be accommodated by a subshell. There are 4 subshells, s, p, d, and f. The s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that. Full Form Of S P D F Subshell.
From www.collegesearch.in
F Orbital Shape Definitions, Orbital Chemistry, Atomic Orbitals Full Form Of S P D F Subshell The n number determines how many of the subshells make up the shell. The maximum number of electrons that can be accommodated by a subshell. Subshells are labelled s, p, d, and f in an electron configuration. How many 2p orbitals are there in an atom? The s subshell has 1 orbital that can hold up to 2 electrons, the. Full Form Of S P D F Subshell.
From www.youtube.com
Full Form Of SST। SST की Full Form क्या है । What Is Full Form Of S.S.T Full Form Of S P D F Subshell The n number determines how many of the subshells make up the shell. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). The labels s, p, d. Full Form Of S P D F Subshell.
From lavelle.chem.ucla.edu
s,p,d,f orbital characteristics? CHEMISTRY COMMUNITY Full Form Of S P D F Subshell The s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up to 10 electrons, and the f. ⚛ group 1 elements occur at the. Subshells are labelled s, p, d, and f in an electron configuration.. Full Form Of S P D F Subshell.
From periodictable.me
nucleus. Dynamic Periodic Table of Elements and Chemistry Full Form Of S P D F Subshell The maximum number of electrons that can be accommodated by a subshell. How would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? How many 2p orbitals are there in an atom? Each subshell can hold a different number of electrons. ⚛ group 1 elements occur at the. A subshell is a subdivision of electron. Full Form Of S P D F Subshell.
From www.youtube.com
DST ka full form Full form of in English Subject DEPARTMENT YouTube Full Form Of S P D F Subshell How many d orbitals must be occupied by single. How would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? The labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. Within each shell of an atom there are some combinations of orbitals. The. Full Form Of S P D F Subshell.
From gbu-taganskij.ru
SPDF Orbitals Explained Quantum Numbers, Electron, 53 OFF Full Form Of S P D F Subshell The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. The maximum number of electrons that can be accommodated by a subshell. Subshells are labelled s, p, d, and f in an electron configuration. Each subshell can hold a different number of electrons. The n number determines how many of. Full Form Of S P D F Subshell.